A Linkage between Angiogenesis and Inflammation in Neovascular Age-Related Macular Degeneration
- PMID: 36359849
- PMCID: PMC9654543
- DOI: 10.3390/cells11213453
A Linkage between Angiogenesis and Inflammation in Neovascular Age-Related Macular Degeneration
Abstract
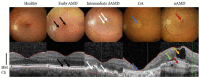
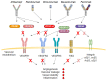
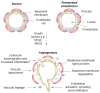
Age-related macular degeneration (AMD) is the leading cause of visual impairment in the aging population with a limited understanding of its pathogenesis and the number of patients are all the time increasing. AMD is classified into two main forms: dry and neovascular AMD (nAMD). Dry AMD is the most prevalent form (80-90%) of AMD cases. Neovascular AMD (10-20% of AMD cases) is treated with monthly or more sparsely given intravitreal anti-vascular endothelial growth factor inhibitors, but unfortunately, not all patients respond to the current treatments. A clinical hallmark of nAMD is choroidal neovascularization. The progression of AMD is initially characterized by atrophic alterations in the retinal pigment epithelium, as well as the formation of lysosomal lipofuscin and extracellular drusen deposits. Cellular damage caused by chronic oxidative stress, protein aggregation and inflammatory processes may lead to advanced geographic atrophy and/or choroidal neovascularization and fibrosis. Currently, it is not fully known why different AMD phenotypes develop. In this review, we connect angiogenesis and inflammatory regulators in the development of nAMD and discuss therapy challenges and hopes.
Keywords: aggregation; aging; angiogenesis; degeneration; inflammation; macula.
Conflict of interest statement
Hanna Heloterä is also an employee of Roche Oy, otherwise, the authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures

Similar articles
-
Evolution of oxidative stress, inflammation and neovascularization in the choroid and retina in a subretinal lipid induced age-related macular degeneration model.Exp Eye Res. 2021 Feb;203:108391. doi: 10.1016/j.exer.2020.108391. Epub 2020 Dec 8. Exp Eye Res. 2021. PMID: 33307075
-
Age-related macular degeneration and myeloproliferative neoplasms - A common pathway.Acta Ophthalmol. 2022 Oct;100 Suppl 271(Suppl 271):3-35. doi: 10.1111/aos.15247. Acta Ophthalmol. 2022. PMID: 36200281 Free PMC article.
-
Emerging Role of Adiponectin/AdipoRs Signaling in Choroidal Neovascularization, Age-Related Macular Degeneration, and Diabetic Retinopathy.Biomolecules. 2023 Jun 13;13(6):982. doi: 10.3390/biom13060982. Biomolecules. 2023. PMID: 37371562 Free PMC article. Review.
-
Histopathology of Age-Related Macular Degeneration and Implications for Pathogenesis and Therapy.Adv Exp Med Biol. 2021;1256:67-88. doi: 10.1007/978-3-030-66014-7_3. Adv Exp Med Biol. 2021. PMID: 33847998
-
Molecular pathogenesis of subretinal fibrosis in neovascular AMD focusing on epithelial-mesenchymal transformation of retinal pigment epithelium.Neurobiol Dis. 2023 Sep;185:106250. doi: 10.1016/j.nbd.2023.106250. Epub 2023 Aug 2. Neurobiol Dis. 2023. PMID: 37536385 Review.
Cited by
-
Revamping anti-cGAS-STING therapy via an injectable thermo-responsive supramolecular hydrogel for pathological retinal angiogenesis.Asian J Pharm Sci. 2024 Oct;19(5):100969. doi: 10.1016/j.ajps.2024.100969. Epub 2024 Sep 21. Asian J Pharm Sci. 2024. PMID: 39474128 Free PMC article.
-
To Treat or Not to Treat? Resolving the Question of Subretinal and Intraretinal Fluid in Age-Related Macular Degeneration: A Narrative Review.Ophthalmol Ther. 2025 Mar;14(3):489-514. doi: 10.1007/s40123-025-01093-3. Epub 2025 Feb 4. Ophthalmol Ther. 2025. PMID: 39904844 Free PMC article. Review.
-
Potential of autophagy in subretinal fibrosis in neovascular age-related macular degeneration.Cell Mol Biol Lett. 2025 Apr 30;30(1):54. doi: 10.1186/s11658-025-00732-8. Cell Mol Biol Lett. 2025. PMID: 40307700 Free PMC article. Review.
-
Dual VEGF-Targeting Strategy Via AAV2-Delivered sFLT-1 and shVEGF for Retinal Neovascularization Therapy.Invest Ophthalmol Vis Sci. 2025 Aug 1;66(11):37. doi: 10.1167/iovs.66.11.37. Invest Ophthalmol Vis Sci. 2025. PMID: 40810574 Free PMC article.
-
Serum RNA Profile Reflects Fluid Status and Atrophic Retinal Changes in Neovascular Age-Related Macular Degeneration.Int J Mol Sci. 2025 May 19;26(10):4852. doi: 10.3390/ijms26104852. Int J Mol Sci. 2025. PMID: 40429992 Free PMC article.
References
-
- Flaxman S.R., Bourne R.R.A., Resnikoff S., Ackland P., Braithwaite T., Cicinelli M.V., Das A., Jonas J.B., Keeffe J., Kempen J.H., et al. Global causes of blindness and distance vision impairment 1990-2020: A systematic review and meta-analysis. Lancet Glob. Health. 2017;5:e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. - DOI - PubMed
-
- Wong W.L., Su X., Li X., Cheung C.M., Klein R., Cheng C.Y., Wong T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health. 2014;2:e106–e116. doi: 10.1016/S2214-109X(13)70145-1. - DOI - PubMed
-
- Corazza P., D’Alterio F.M., Kabbani J., Alam M.M.R., Mercuri S., Orlans H.O., Younis S. Long-term outcomes of intravitreal anti-vegf therapies in patients affected by neovascular age-related macular degeneration: A real-life study. BMC Ophthalmol. 2021;21:300. doi: 10.1186/s12886-021-02055-6. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

