Analysis of Homologous Regions of Small RNAs MIR397 and MIR408 Reveals the Conservation of Microsynteny among Rice Crop-Wild Relatives
- PMID: 36359857
- PMCID: PMC9656352
- DOI: 10.3390/cells11213461
Analysis of Homologous Regions of Small RNAs MIR397 and MIR408 Reveals the Conservation of Microsynteny among Rice Crop-Wild Relatives
Abstract
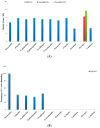
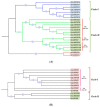
MIRNAs are small non-coding RNAs that play important roles in a wide range of biological processes in plant growth and development. MIR397 (involved in drought, low temperature, and nitrogen and copper (Cu) starvation) and MIR408 (differentially expressed in response to environmental stresses such as copper, light, mechanical stress, dehydration, cold, reactive oxygen species, and drought) belong to conserved MIRNA families that either negatively or positively regulate their target genes. In the present study, we identified the homologs of MIR397 and MIR408 in Oryza sativa and its six wild progenitors, three non-Oryza species, and one dicot species. We analyzed the 100 kb segments harboring MIRNA homologs from 11 genomes to obtain a comprehensive view of their community evolution around these loci in the farthest (distant) relatives of rice. Our study showed that mature MIR397 and MIR408 were highly conserved among all Oryza species. Comparative genomics analyses also revealed that the microsynteny of the 100 kb region surrounding MIRNAs was only conserved in Oryza spp.; disrupted in Sorghum, maize, and wheat; and completely lost in Arabidopsis. There were deletions, rearrangements, and translocations within the 100 kb segments in Oryza spp., but the overall microsynteny of the region was maintained. The phylogenetic analyses of the precursor regions of all MIRNAs under study revealed a bimodal clade of common origin. This comparative analysis of miRNA involved in abiotic stress tolerance in plants provides a powerful tool for future Oryza research. Crop wild relatives (CWRs) offer multiple traits with potential to decrease the amount of yield loss owing to biotic and abiotic stresses. Using a comparative genomics approach, the exploration of CWRs as a source of tolerance to these stresses by understanding their evolution can be further used to leverage their yield potential.
Keywords: MIR397; MIR408; MIRNAs; Oryza; comparative genomics; crop wild relatives; microsynteny.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











References
-
- Shanchez P., Wing R.A., Brar D. In: Genetics and Genomics of Rice. Zhang Q., Wing R., editors. Springer Science+Business Media, LLC; Berlin, Germany: 2013.
-
- Shakiba E., Eizenga G. In: Rice-Germplasm, Genetics and Improvement. Bao J., editor. IntechOpen; London, UK: 2014.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

