Src Family Kinases Facilitate the Crosstalk between CGRP and Cytokines in Sensitizing Trigeminal Ganglion via Transmitting CGRP Receptor/PKA Pathway
- PMID: 36359895
- PMCID: PMC9655983
- DOI: 10.3390/cells11213498
Src Family Kinases Facilitate the Crosstalk between CGRP and Cytokines in Sensitizing Trigeminal Ganglion via Transmitting CGRP Receptor/PKA Pathway
Abstract
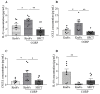
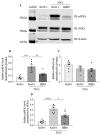
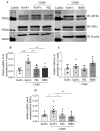
The communication between calcitonin gene-related peptide (CGRP) and cytokines plays a prominent role in maintaining trigeminal ganglion (TG) and trigeminovascular sensitization. However, the underlying regulatory mechanism is elusive. In this study, we explored the hypothesis that Src family kinases (SFKs) activity facilitates the crosstalk between CGRP and cytokines in sensitizing TG. Mouse TG tissue culture was performed to study CGRP release by enzyme-linked immunosorbent assay, cytokine release by multiplex assay, cytokine gene expression by quantitative polymerase chain reaction, and phosphorylated SFKs level by western blot. The results demonstrated that a SFKs activator, pYEEI (YGRKKRRQRRREPQY(PO3H2)EEIPIYL) alone, did not alter CGRP release or the inflammatory cytokine interleukin-1β (IL-1β) gene expression in the mouse TG. In contrast, a SFKs inhibitor, saracatinib, restored CGRP release, the inflammatory cytokines IL-1β, C-X-C motif ligand 1, C-C motif ligand 2 (CCL2) release, and IL-1β, CCL2 gene expression when the mouse TG was pre-sensitized with hydrogen peroxide and CGRP respectively. Consistently with this, the phosphorylated SFKs level was increased by both hydrogen peroxide and CGRP in the mouse TG, which was reduced by a CGRP receptor inhibitor BIBN4096 and a protein kinase A (PKA) inhibitor PKI (14-22) Amide. The present study demonstrates that SFKs activity plays a pivotal role in facilitating the crosstalk between CGRP and cytokines by transmitting CGRP receptor/PKA signaling to potentiate TG sensitization and ultimately trigeminovascular sensitization.
Keywords: C-C motif ligand 2; C-X-C motif ligand 1; Src family kinases; calcitonin gene-related peptide; interleukin-1β; migraine; protein kinase A; trigeminal ganglion.
Conflict of interest statement
The authors declare no potential conflict of interest with respect to the research, authorship, and/or publication of this article.
Figures






Similar articles
-
TRPA1-Mediated Src Family Kinases Activity Facilitates Cortical Spreading Depression Susceptibility and Trigeminovascular System Sensitization.Int J Mol Sci. 2021 Nov 12;22(22):12273. doi: 10.3390/ijms222212273. Int J Mol Sci. 2021. PMID: 34830154 Free PMC article.
-
Mitogen-activated protein kinase pathways are involved in the upregulation of calcitonin gene-related peptide of rat trigeminal ganglion after organ culture.J Mol Neurosci. 2012 Sep;48(1):53-65. doi: 10.1007/s12031-012-9772-y. Epub 2012 Apr 20. J Mol Neurosci. 2012. PMID: 22528462
-
Elevated levels of calcitonin gene-related peptide in upper spinal cord promotes sensitization of primary trigeminal nociceptive neurons.Neuroscience. 2016 Dec 17;339:491-501. doi: 10.1016/j.neuroscience.2016.10.013. Epub 2016 Oct 13. Neuroscience. 2016. PMID: 27746346 Free PMC article.
-
The Trigeminovascular Pathway: Role of CGRP and CGRP Receptors in Migraine.Headache. 2017 May;57 Suppl 2:47-55. doi: 10.1111/head.13081. Headache. 2017. PMID: 28485848 Review.
-
Current understanding of trigeminal ganglion structure and function in headache.Cephalalgia. 2019 Nov;39(13):1661-1674. doi: 10.1177/0333102418786261. Epub 2018 Jul 10. Cephalalgia. 2019. PMID: 29989427 Free PMC article. Review.
Cited by
-
Exploring Novel Therapeutic Targets in the Common Pathogenic Factors in Migraine and Neuropathic Pain.Int J Mol Sci. 2023 Feb 18;24(4):4114. doi: 10.3390/ijms24044114. Int J Mol Sci. 2023. PMID: 36835524 Free PMC article. Review.
-
Gender-different effect of Src family kinases antagonism on photophobia and trigeminal ganglion activity.J Headache Pain. 2024 Oct 11;25(1):175. doi: 10.1186/s10194-024-01875-3. J Headache Pain. 2024. PMID: 39390364 Free PMC article.
-
Navigating the Neurobiology of Migraine: From Pathways to Potential Therapies.Cells. 2024 Jun 25;13(13):1098. doi: 10.3390/cells13131098. Cells. 2024. PMID: 38994951 Free PMC article.
-
Method for cryopreservation of trigeminal ganglion for establishing primary cultures of neurons and glia.J Neurosci Methods. 2024 Feb;402:110034. doi: 10.1016/j.jneumeth.2023.110034. Epub 2023 Dec 10. J Neurosci Methods. 2024. PMID: 38072069 Free PMC article.
-
Biomarkers of Migraine: An Integrated Evaluation of Preclinical and Clinical Findings.Int J Mol Sci. 2023 Mar 10;24(6):5334. doi: 10.3390/ijms24065334. Int J Mol Sci. 2023. PMID: 36982428 Free PMC article. Review.
References
-
- Ray B.S., Wolff H.G. Experimental studies on headache: Pain-sensitive structures of the head and their significance in heaadache. Arch. Surg. 1940;41:813–856. doi: 10.1001/archsurg.1940.01210040002001. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

