A Balance between Transmembrane-Mediated ER/Golgi Retention and Forward Trafficking Signals in Glycophorin-Anion Exchanger-1 Interaction
- PMID: 36359907
- PMCID: PMC9653601
- DOI: 10.3390/cells11213512
A Balance between Transmembrane-Mediated ER/Golgi Retention and Forward Trafficking Signals in Glycophorin-Anion Exchanger-1 Interaction
Abstract
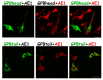
Anion exchanger-1 (AE1) is the main erythroid Cl-/HCO3- transporter that supports CO2 transport. Glycophorin A (GPA), a component of the AE1 complexes, facilitates AE1 expression and anion transport, but Glycophorin B (GPB) does not. Here, we dissected the structural components of GPA/GPB involved in glycophorin-AE1 trafficking by comparing them with three GPB variants-GPBhead (lacking the transmembrane domain [TMD]), GPBtail (mainly the TMD), and GP.Mur (glycophorin B-A-B hybrid). GPB-derived GP.Mur bears an O-glycopeptide that encompasses the R18 epitope, which is present in GPA but not GPB. By flow cytometry, AE1 expression in the control erythrocytes increased with the GPA-R18 expression; GYP.Mur+/+ erythrocytes bearing both GP.Mur and GPA expressed more R18 epitopes and more AE1 proteins. In contrast, heterologously expressed GPBtail and GPB were predominantly localized in the Golgi apparatus of HEK-293 cells, whereas GBhead was diffuse throughout the cytosol, suggesting that glycophorin transmembrane encoded an ER/Golgi retention signal. AE1 coexpression could reduce the ER/Golgi retention of GPB, but not of GPBtail or GPBhead. Thus, there are forward-trafficking and transmembrane-driven ER/Golgi retention signals encoded in the glycophorin sequences. How the balance between these opposite trafficking signals could affect glycophorin sorting into AE1 complexes and influence erythroid anion transport remains to be explored.
Keywords: ER/Golgi retention; GP.Mur (Miltenberger subtype III; Mi.III); SLC4A1); anion exchanger-1 (AE1; band 3; erythrocytes); glycophorin A (GPA); glycophorin B (GPB); membrane protein; oligomerization; red blood cells (RBCs; trafficking; transmembrane domain (TMD).
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
Miltenberger blood group antigen type III (Mi.III) enhances the expression of band 3.Blood. 2009 Aug 27;114(9):1919-28. doi: 10.1182/blood-2008-12-195180. Epub 2009 Jun 29. Blood. 2009. PMID: 19564639 Free PMC article.
-
Distinct regions of human glycophorin A enhance human red cell anion exchanger (band 3; AE1) transport function and surface trafficking.J Biol Chem. 2003 Aug 29;278(35):32954-61. doi: 10.1074/jbc.M302527200. Epub 2003 Jun 17. J Biol Chem. 2003. PMID: 12813056
-
Differential enzymatic deglycosylation reveals attachment of red cell B antigen onto the carbohydrate moiety of glycophorin A and glycophorin B.Vox Sang. 2023 Feb;118(2):147-152. doi: 10.1111/vox.13385. Epub 2022 Dec 12. Vox Sang. 2023. PMID: 36510386
-
The major integral proteins of the human red cell.Baillieres Clin Haematol. 1993 Jun;6(2):333-56. doi: 10.1016/s0950-3536(05)80149-0. Baillieres Clin Haematol. 1993. PMID: 8043929 Review.
-
[Integral proteins of red cell membranes: their genetic and phenotypic expressions].Nihon Rinsho. 1996 Sep;54(9):2348-63. Nihon Rinsho. 1996. PMID: 8890562 Review. Japanese.
Cited by
-
Role of channels in the O2 permeability of murine red blood cells II. Morphological and proteomic studies.bioRxiv [Preprint]. 2025 May 18:2025.03.05.639962. doi: 10.1101/2025.03.05.639962. bioRxiv. 2025. PMID: 40462928 Free PMC article. Preprint.
-
Increased Anion Exchanger-1 (Band 3) on the Red Blood Cell Membrane Accelerates Scavenging of Nitric Oxide Metabolites and Predisposes Hypertension Risks.Function (Oxf). 2025 Feb 12;6(1):zqae052. doi: 10.1093/function/zqae052. Function (Oxf). 2025. PMID: 39656872 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

