The Potential for Placental Activation of PPARγ to Improve the Angiogenic Profile in Preeclampsia
- PMID: 36359910
- PMCID: PMC9659243
- DOI: 10.3390/cells11213514
The Potential for Placental Activation of PPARγ to Improve the Angiogenic Profile in Preeclampsia
Abstract
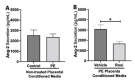
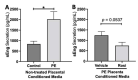
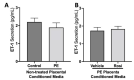
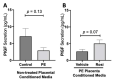
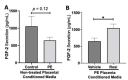
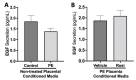
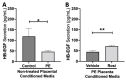
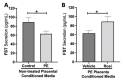
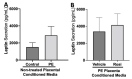
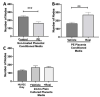
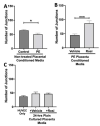
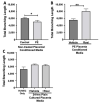
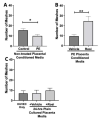
Preeclampsia (PE) is one of the most common causes of maternal-fetal morbidity and mortality world-wide. While the underlying causes of PE remain elusive, aberrant trophoblast differentiation and function are thought to cause an imbalance of secreted angiogenic proteins resulting in systemic endothelial dysfunction and organ damage in the mother. The placental dysfunction is also characterized by a reduction of the transcription factor, peroxisome proliferator activated receptor γ (PPARγ) which normally promotes trophoblast differentiation and healthy placental function. This study aimed to understand how placental activation of PPARγ effects the secretion of angiogenic proteins and subsequently endothelial function. To study this, healthy and PE placental tissues were cultured with or without the PPARγ agonist, Rosiglitazone, and a Luminex assay was performed to measure secreted proteins from the placenta. To assess the angiogenic effects of placental activation of PPARγ, human umbilical vein endothelial cells (HUVECs) were cultured with the placental conditioned media and the net angiogenic potential of these cells was measured by a tube formation assay. This is the first study to show PPARγ's beneficial effect on the angiogenic profile in the human preeclamptic placenta through the reduction of anti-angiogenic angiopoietin-2 and soluble endoglin and the upregulation of pro-angiogenic placental growth factor, fibroblast growth factor-2, heparin-binding epidermal growth factor, and follistatin. The changes in the angiogenic profile were supported by the increased angiogenic potential observed in the HUVECs when cultured with conditioned media from rosiglitazone-treated preeclamptic placentas. The restoration of these disrupted pathways by activation of PPARγ in the preeclamptic placenta offers potential to improve placental and endothelial function in PE.
Keywords: PPARγ; angiogenesis; placenta; preeclampsia.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Rosiglitazone-Mediated Activation of PPARγ Restores HO1 Expression in the Human Preeclamptic Placenta.Hypertension. 2023 Nov;80(11):2386-2396. doi: 10.1161/HYPERTENSIONAHA.123.21645. Epub 2023 Sep 13. Hypertension. 2023. PMID: 37702083 Free PMC article.
-
Procyanidin B2 ameliorates endothelial dysfunction and impaired angiogenesis via the Nrf2/PPARγ/sFlt-1 axis in preeclampsia.Pharmacol Res. 2022 Mar;177:106127. doi: 10.1016/j.phrs.2022.106127. Epub 2022 Feb 10. Pharmacol Res. 2022. PMID: 35150862
-
Rosiglitazone-Mediated Activation of PPARγ Induces PlGF Expression in Trophoblast Cells.Reprod Sci. 2025 Jun;32(6):1864-1875. doi: 10.1007/s43032-025-01868-w. Epub 2025 Apr 28. Reprod Sci. 2025. PMID: 40295383 Free PMC article.
-
[Clinical value of angiogenic and anti-angiogenic marker assay in preeclampsia].Rev Med Suisse. 2020 Oct 28;16(712):2031-2036. Rev Med Suisse. 2020. PMID: 33112515 Review. French.
-
Angiogenic Imbalance as a Contributor of Preeclampsia.Curr Pharm Biotechnol. 2018;19(10):797-815. doi: 10.2174/1389201019666180925115559. Curr Pharm Biotechnol. 2018. PMID: 30255753 Review.
Cited by
-
Recent Insights into the Role of PPARs in Disease.Cells. 2023 Jun 7;12(12):1572. doi: 10.3390/cells12121572. Cells. 2023. PMID: 37371042 Free PMC article.
-
PPARG-centric transcriptional re-wiring during differentiation of human trophoblast stem cells into extravillous trophoblasts.Nucleic Acids Res. 2025 Jul 19;53(14):gkaf669. doi: 10.1093/nar/gkaf669. Nucleic Acids Res. 2025. PMID: 40705926 Free PMC article.
-
The Role of Peroxisome Proliferator-Activated Receptors in Preeclampsia.Cells. 2023 Feb 17;12(4):647. doi: 10.3390/cells12040647. Cells. 2023. PMID: 36831316 Free PMC article. Review.
-
Rosiglitazone-Mediated Activation of PPARγ Restores HO1 Expression in the Human Preeclamptic Placenta.Hypertension. 2023 Nov;80(11):2386-2396. doi: 10.1161/HYPERTENSIONAHA.123.21645. Epub 2023 Sep 13. Hypertension. 2023. PMID: 37702083 Free PMC article.
-
The NFκB Signaling Pathway Is Involved in the Pathophysiological Process of Preeclampsia.Geburtshilfe Frauenheilkd. 2024 Apr 10;84(4):334-345. doi: 10.1055/a-2273-6318. eCollection 2024 Apr. Geburtshilfe Frauenheilkd. 2024. PMID: 38618576 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

