Generation of Lens Progenitor Cells and Lentoid Bodies from Pluripotent Stem Cells: Novel Tools for Human Lens Development and Ocular Disease Etiology
- PMID: 36359912
- PMCID: PMC9658148
- DOI: 10.3390/cells11213516
Generation of Lens Progenitor Cells and Lentoid Bodies from Pluripotent Stem Cells: Novel Tools for Human Lens Development and Ocular Disease Etiology
Abstract
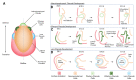
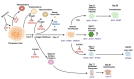
In vitro differentiation of human pluripotent stem cells (hPSCs) into specialized tissues and organs represents a powerful approach to gain insight into those cellular and molecular mechanisms regulating human development. Although normal embryonic eye development is a complex process, generation of ocular organoids and specific ocular tissues from pluripotent stem cells has provided invaluable insights into the formation of lineage-committed progenitor cell populations, signal transduction pathways, and self-organization principles. This review provides a comprehensive summary of recent advances in generation of adenohypophyseal, olfactory, and lens placodes, lens progenitor cells and three-dimensional (3D) primitive lenses, "lentoid bodies", and "micro-lenses". These cells are produced alone or "community-grown" with other ocular tissues. Lentoid bodies/micro-lenses generated from human patients carrying mutations in crystallin genes demonstrate proof-of-principle that these cells are suitable for mechanistic studies of cataractogenesis. Taken together, current and emerging advanced in vitro differentiation methods pave the road to understand molecular mechanisms of cataract formation caused by the entire spectrum of mutations in DNA-binding regulatory genes, such as PAX6, SOX2, FOXE3, MAF, PITX3, and HSF4, individual crystallins, and other genes such as BFSP1, BFSP2, EPHA2, GJA3, GJA8, LIM2, MIP, and TDRD7 represented in human cataract patients.
Keywords: PAX6; cranial placodes; crystallins; de-nucleation; differentiation; gene expression; lens progenitor cells; lentoid bodies; optic cup; pluripotent stem cells; self-organization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Efficient generation of lens progenitor cells and lentoid bodies from human embryonic stem cells in chemically defined conditions.FASEB J. 2010 Sep;24(9):3274-83. doi: 10.1096/fj.10-157255. Epub 2010 Apr 21. FASEB J. 2010. PMID: 20410439 Free PMC article.
-
Generation of Functional Lentoid Bodies From Human Induced Pluripotent Stem Cells Derived From Urinary Cells.Invest Ophthalmol Vis Sci. 2017 Jan 1;58(1):517-527. doi: 10.1167/iovs.16-20504. Invest Ophthalmol Vis Sci. 2017. PMID: 28125839
-
Molecular characterization of the human lens epithelium-derived cell line SRA01/04.Exp Eye Res. 2019 Nov;188:107787. doi: 10.1016/j.exer.2019.107787. Epub 2019 Aug 31. Exp Eye Res. 2019. PMID: 31479653 Free PMC article.
-
Lens Development and Crystallin Gene Expression.Prog Mol Biol Transl Sci. 2015;134:129-67. doi: 10.1016/bs.pmbts.2015.05.001. Epub 2015 Jun 12. Prog Mol Biol Transl Sci. 2015. PMID: 26310154 Review.
-
Crosstalk between signaling pathways (Rho/ROCK, TGF-β and Wnt/β-Catenin Pathways/ PI3K-AKT-mTOR) in Cataract: A Mechanistic Exploration and therapeutic strategy.Gene. 2025 May 5;947:149338. doi: 10.1016/j.gene.2025.149338. Epub 2025 Feb 16. Gene. 2025. PMID: 39965745 Review.
Cited by
-
Biologically Relevant Laminin-511 Moderates the Derivation and Proliferation of Human Lens Epithelial Stem/Progenitor-Like Cells.Invest Ophthalmol Vis Sci. 2024 Aug 1;65(10):12. doi: 10.1167/iovs.65.10.12. Invest Ophthalmol Vis Sci. 2024. PMID: 39106056 Free PMC article.
-
Advancements in Ocular Regenerative Therapies.Biology (Basel). 2023 May 19;12(5):737. doi: 10.3390/biology12050737. Biology (Basel). 2023. PMID: 37237549 Free PMC article. Review.
-
Differentiation of mesenchymal stem cells towards lens epithelial stem cells based on three-dimensional bio-printed matrix.Front Cell Dev Biol. 2025 Jan 6;12:1526943. doi: 10.3389/fcell.2024.1526943. eCollection 2024. Front Cell Dev Biol. 2025. PMID: 39834393 Free PMC article.
-
Intermediate filaments and their associated molecules.J Biomed Res. 2025 Feb 8;39(3):242-253. doi: 10.7555/JBR.38.20240193. J Biomed Res. 2025. PMID: 39930669 Free PMC article.
-
Eye Lens Organoids Made Simple: Characterization of a New Three-Dimensional Organoid Model for Lens Development and Pathology.Cells. 2023 Oct 18;12(20):2478. doi: 10.3390/cells12202478. Cells. 2023. PMID: 37887322 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

