PI3K Isoform-Specific Regulation of Leader and Follower Cell Function for Collective Migration and Proliferation in Response to Injury
- PMID: 36359913
- PMCID: PMC9658457
- DOI: 10.3390/cells11213515
PI3K Isoform-Specific Regulation of Leader and Follower Cell Function for Collective Migration and Proliferation in Response to Injury
Abstract
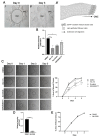
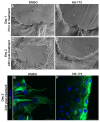
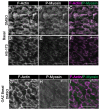
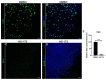
To ensure proper wound healing it is important to elucidate the signaling cues that coordinate leader and follower cell behavior to promote collective migration and proliferation for wound healing in response to injury. Using an ex vivo post-cataract surgery wound healing model we investigated the role of class I phosphatidylinositol-3-kinase (PI3K) isoforms in this process. Our findings revealed a specific role for p110α signaling independent of Akt for promoting the collective migration and proliferation of the epithelium for wound closure. In addition, we found an important role for p110α signaling in orchestrating proper polarized cytoskeletal organization within both leader and wounded epithelial follower cells to coordinate their function for wound healing. p110α was necessary to signal the formation and persistence of vimentin rich-lamellipodia extensions by leader cells and the reorganization of actomyosin into stress fibers along the basal domains of the wounded lens epithelial follower cells for movement. Together, our study reveals a critical role for p110α in the collective migration of an epithelium in response to wounding.
Keywords: PI3K; collective migration; follower cell; leader cell; p110α; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

