Arginine Reduces Glycation in γ2 Subunit of AMPK and Pathologies in Alzheimer's Disease Model Mice
- PMID: 36359916
- PMCID: PMC9655994
- DOI: 10.3390/cells11213520
Arginine Reduces Glycation in γ2 Subunit of AMPK and Pathologies in Alzheimer's Disease Model Mice
Abstract
The metabolism disorders are a common convergence of Alzheimer's disease (AD) and type 2 diabetes mellitus (T2DM). The characteristics of AD are senile plaques and neurofibrillary tangles (NFTs) composed by deposits of amyloid-β (Aβ) and phosphorylated tau, respectively. Advanced glycation end-products (AGEs) are a stable modification of proteins by non-enzymatic reactions, which could result in the protein dysfunction. AGEs are associated with some disease developments, such as diabetes mellitus and AD, but the effects of the glycated γ2 subunit of AMPK on its activity and the roles in AD onset are unknown.
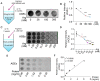
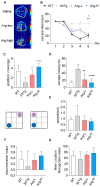
Methods: We studied the effect of glycated γ2 subunit of AMPK on its activity in N2a cells. In 3 × Tg mice, we administrated L-arginine once every two days for 45 days and evaluated the glycation level of γ2 subunit and function of AMPK and alternation of pathologies.
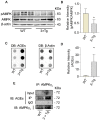
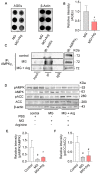
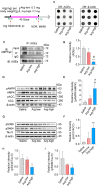
Results: The glycation level of γ2 subunit was significantly elevated in 3 × Tg mice as compared with control mice, meanwhile, the level of pT172-AMPK was obviously lower in 3 × Tg mice than that in control mice. Moreover, we found that arginine protects the γ2 subunit of AMPK from glycation, preserves AMPK function, and improves pathologies and cognitive deficits in 3 × Tg mice.
Conclusions: Arginine treatment decreases glycated γ2 subunit of AMPK and increases p-AMPK levels in 3 × Tg mice, suggesting that reduced glycation of the γ2 subunit could ameliorate AMPK function and become a new target for AD therapy in the future.
Keywords: AMPK; Alzheimer’s disease; L−arginine; advanced glycation end−products; glycation.
Conflict of interest statement
The authors declare that research was conducted in the absent of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures
Similar articles
-
Advanced glycation end products in Alzheimer's disease and other neurodegenerative diseases.Am J Pathol. 1998 Oct;153(4):1149-55. doi: 10.1016/S0002-9440(10)65659-3. Am J Pathol. 1998. PMID: 9777946 Free PMC article.
-
Serum or cerebrospinal fluid levels of glyceraldehyde-derived advanced glycation end products (AGEs) may be a promising biomarker for early detection of Alzheimer's disease.Med Hypotheses. 2005;64(6):1205-7. doi: 10.1016/j.mehy.2005.01.016. Med Hypotheses. 2005. PMID: 15823718
-
AdipoRon improves cognitive dysfunction of Alzheimer's disease and rescues impaired neural stem cell proliferation through AdipoR1/AMPK pathway.Exp Neurol. 2020 May;327:113249. doi: 10.1016/j.expneurol.2020.113249. Epub 2020 Feb 15. Exp Neurol. 2020. PMID: 32070713
-
AMPK: A bridge between diabetes mellitus and Alzheimer's disease.Behav Brain Res. 2021 Feb 26;400:113043. doi: 10.1016/j.bbr.2020.113043. Epub 2020 Dec 8. Behav Brain Res. 2021. PMID: 33307136 Review.
-
Investigations on oxidative stress and therapeutical implications in dementia.Eur Arch Psychiatry Clin Neurosci. 1999;249 Suppl 3:68-73. doi: 10.1007/pl00014177. Eur Arch Psychiatry Clin Neurosci. 1999. PMID: 10654103 Review.
Cited by
-
The metabolic sensor AMPK: Twelve enzymes in one.Mol Metab. 2024 Dec;90:102042. doi: 10.1016/j.molmet.2024.102042. Epub 2024 Oct 2. Mol Metab. 2024. PMID: 39362600 Free PMC article. Review.
-
Chuanxiong Renshen Decoction Inhibits Alzheimer's Disease Neuroinflammation by Regulating PPARγ/NF-κB Pathway.Drug Des Devel Ther. 2024 Jul 24;18:3209-3232. doi: 10.2147/DDDT.S462266. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 39071817 Free PMC article.
-
Development and Assessment of a Prediction Model for Alzheimer's Disease Diagnosis Based on Thermoregulation-Related Genes.Comb Chem High Throughput Screen. 2025;28(6):944-962. doi: 10.2174/0113862073291279240409035856. Comb Chem High Throughput Screen. 2025. PMID: 38747223
-
Amino acid metabolism in health and disease.Signal Transduct Target Ther. 2023 Sep 13;8(1):345. doi: 10.1038/s41392-023-01569-3. Signal Transduct Target Ther. 2023. PMID: 37699892 Free PMC article. Review.
References
-
- Arnold S.E., Arvanitakis Z., Macauley-Rambach S.L., Koenig A.M., Wang H.-Y., Ahima R.S., Craft S., Gandy S., Buettner C., Stoeckel L.E., et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018;14:168–181. doi: 10.1038/nrneurol.2017.185. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

