Neutrophil Extracellular Traps in Asthma: Friends or Foes?
- PMID: 36359917
- PMCID: PMC9654069
- DOI: 10.3390/cells11213521
Neutrophil Extracellular Traps in Asthma: Friends or Foes?
Abstract
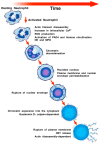
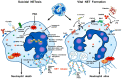
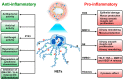
Asthma is a chronic inflammatory disease characterized by variable airflow limitation and airway hyperresponsiveness. A plethora of immune and structural cells are involved in asthma pathogenesis. The roles of neutrophils and their mediators in different asthma phenotypes are largely unknown. Neutrophil extracellular traps (NETs) are net-like structures composed of DNA scaffolds, histones and granular proteins released by activated neutrophils. NETs were originally described as a process to entrap and kill a variety of microorganisms. NET formation can be achieved through a cell-death process, termed NETosis, or in association with the release of DNA from viable neutrophils. NETs can also promote the resolution of inflammation by degrading cytokines and chemokines. NETs have been implicated in the pathogenesis of various non-infectious conditions, including autoimmunity, cancer and even allergic disorders. Putative surrogate NET biomarkers (e.g., double-strand DNA (dsDNA), myeloperoxidase-DNA (MPO-DNA), and citrullinated histone H3 (CitH3)) have been found in different sites/fluids of patients with asthma. Targeting NETs has been proposed as a therapeutic strategy in several diseases. However, different NETs and NET components may have alternate, even opposite, consequences on inflammation. Here we review recent findings emphasizing the pathogenic and therapeutic potential of NETs in asthma.
Keywords: NETs; PMN; TSLP; VEGF; asthma; inflammation; neutrophil extracellular traps; neutrophils.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Neutrophil extracellular traps and neutrophil-related mediators in human thyroid cancer.Front Immunol. 2023 Aug 29;14:1167404. doi: 10.3389/fimmu.2023.1167404. eCollection 2023. Front Immunol. 2023. PMID: 37705974 Free PMC article.
-
Simvastatin Reduces NETosis to Attenuate Severe Asthma by Inhibiting PAD4 Expression.Oxid Med Cell Longev. 2023 Feb 2;2023:1493684. doi: 10.1155/2023/1493684. eCollection 2023. Oxid Med Cell Longev. 2023. PMID: 36778209 Free PMC article.
-
NETopathic Inflammation in Chronic Obstructive Pulmonary Disease and Severe Asthma.Front Immunol. 2019 Feb 5;10:47. doi: 10.3389/fimmu.2019.00047. eCollection 2019. Front Immunol. 2019. PMID: 30804927 Free PMC article. Review.
-
Neutrophil extracellular traps and neutrophil-derived mediators as possible biomarkers in bronchial asthma.Clin Exp Med. 2022 May;22(2):285-300. doi: 10.1007/s10238-021-00750-8. Epub 2021 Aug 3. Clin Exp Med. 2022. PMID: 34342773 Free PMC article.
-
Role of Neutrophil Extracellular Traps in Asthma and Chronic Obstructive Pulmonary Disease.Chin Med J (Engl). 2017 Mar 20;130(6):730-736. doi: 10.4103/0366-6999.201608. Chin Med J (Engl). 2017. PMID: 28303858 Free PMC article. Review.
Cited by
-
Altered expression of long noncoding RNAs regulating neutrophilic inflammation in peripheral blood was associated with symptom severity in patients with house dust mite-induced allergic rhinitis.Front Allergy. 2024 Oct 25;5:1466480. doi: 10.3389/falgy.2024.1466480. eCollection 2024. Front Allergy. 2024. PMID: 39525400 Free PMC article.
-
Study on identification and analysis of biomarkers related to neutrophils extracellular traps in childhood asthma.Medicine (Baltimore). 2025 Aug 1;104(31):e43489. doi: 10.1097/MD.0000000000043489. Medicine (Baltimore). 2025. PMID: 40760602 Free PMC article.
-
Transcriptome analysis reveals the impact of NETs activation on airway epithelial cell EMT and inflammation in bronchiolitis obliterans.Sci Rep. 2023 Nov 6;13(1):19226. doi: 10.1038/s41598-023-45617-y. Sci Rep. 2023. PMID: 37932341 Free PMC article.
-
Persistent neutrophilic inflammation is associated with delayed toxicity of phenylarsine oxide in lungs.Sci Rep. 2025 Apr 7;15(1):11840. doi: 10.1038/s41598-025-95645-z. Sci Rep. 2025. PMID: 40195488 Free PMC article.
-
Extracellular Traps in Inflammation: Pathways and Therapeutic Targets.Life (Basel). 2025 Apr 8;15(4):627. doi: 10.3390/life15040627. Life (Basel). 2025. PMID: 40283181 Free PMC article. Review.
References
-
- Khalfaoui L., Symon F.A., Couillard S., Hargadon B., Chaudhuri R., Bicknell S., Mansur A.H., Shrimanker R., Hinks T.S.C., Pavord I.D., et al. Airway remodelling rather than cellular infiltration characterizes both type2 cytokine biomarker-high and -low severe asthma. Allergy. 2022;77:2974. doi: 10.1111/all.15376. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

