Branched-Chain Amino Acids Are Linked with Alzheimer's Disease-Related Pathology and Cognitive Deficits
- PMID: 36359919
- PMCID: PMC9658564
- DOI: 10.3390/cells11213523
Branched-Chain Amino Acids Are Linked with Alzheimer's Disease-Related Pathology and Cognitive Deficits
Abstract
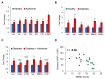
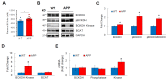
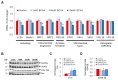
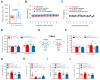
Alzheimer's disease (AD) is an irreversible neurodegenerative disorder with a complex pathophysiology. Type 2 diabetes (T2D) is a strong risk factor for AD that shares similar abnormal features including metabolic dysregulation and brain pathology such as amyloid and/or Tau deposits. Emerging evidence suggests that circulating branched-chain amino acids (BCAAs) are associated with T2D. While excess BCAAs are shown to be harmful to neurons, its connection to AD is poorly understood. Here we show that individuals with AD have elevated circulating BCAAs and their metabolites compared to healthy individuals, and that a BCAA metabolite is correlated with the severity of dementia. APPSwe mouse model of AD also displayed higher plasma BCAAs compared to controls. In pursuit of understanding a potential causality, BCAA supplementation to HT-22 neurons was found to reduce genes critical for neuronal health while increasing phosphorylated Tau. Moreover, restricting BCAAs from diet delayed cognitive decline and lowered AD-related pathology in the cortex and hippocampus in APP/PS1 mice. BCAA restriction for two months was sufficient to correct glycemic control and increased/restored dopamine that were severely reduced in APP/PS1 controls. Treating 5xFAD mice that show early brain pathology with a BCAA-lowering compound recapitulated the beneficial effects of BCAA restriction on brain pathology and neurotransmitters including norepinephrine and serotonin. Collectively, this study reveals a positive association between circulating BCAAs and AD. Our findings suggest that BCAAs impair neuronal functions whereas BCAA-lowering alleviates AD-related pathology and cognitive decline, thus establishing a potential causal link between BCAAs and AD progression.
Keywords: 5xFAD; APP/PS1; Aβ-42; BCAA; Tau; diet restriction; glucose metabolism; neurotransmitters.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Alzheimer’s Association Alzheimer’s Disease Facts and Figures. [(accessed on 8 September 2022)]. Available online: https://www.alz.org/alzheimers-dementia/facts-figures.
-
- Adams S.H., Hoppel C.L., Lok K.H., Zhao L., Wong S.W., Minkler P.E., Hwang D.H., Newman J.W., Garvey W.T. Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid beta-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J. Nutr. 2009;139:1073–1081. doi: 10.3945/jn.108.103754. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

