Involvement of NINJ2 Protein in Inflammation and Blood-Brain Barrier Transmigration of Monocytes in Multiple Sclerosis
- PMID: 36360183
- PMCID: PMC9690398
- DOI: 10.3390/genes13111946
Involvement of NINJ2 Protein in Inflammation and Blood-Brain Barrier Transmigration of Monocytes in Multiple Sclerosis
Abstract
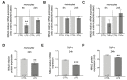
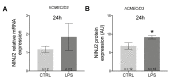
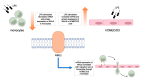
Multiple sclerosis (MS) is an inflammatory neurodegenerative disorder of the central nervous system (CNS). The migration of immune cells into the CNS is essential for its development, and plasma membrane molecules play an important role in triggering and maintaining the inflammation. We previously identified ninjurin2, a plasma membrane protein encoded by NINJ2 gene, as involved in the occurrence of relapse under Interferon-β treatment in MS patients. The aim of the present study was to investigate the involvement of NINJ2 in inflammatory conditions and in the migration of monocytes through the blood-brain barrier (BBB). We observed that NINJ2 is downregulated in monocytes and in THP-1 cells after stimulation with the pro-inflammatory cytokine LPS, while in hCMEC/D3 cells, which represent a surrogate of the BBB, LPS stimulation increases its expression. We set up a transmigration assay using an hCMEC/D3 transwell-based model, finding a higher transmigration rate of monocytes from MS subjects compared to healthy controls (HCs) in the case of an activated hCMEC/D3 monolayer. Moreover, a positive correlation between NINJ2 expression in monocytes and monocyte migration rate was observed. Overall, our results suggest that ninjurin2 could be involved in the transmigration of immune cells into the CNS in pro-inflammatory conditions. Further experiments are needed to elucidate the exact molecular mechanisms.
Keywords: inflammation; multiple sclerosis; transmigration.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Polman C.H., O’Connor P.W., Havrdova E., Hutchinson M., Kappos L., Miller D.H., Phillips J.T., Lublin F.D., Giovannoni G., Wajgt A., et al. A Randomized, Placebo-Controlled Trial of Natalizumab for Relapsing Multiple Sclerosis. New Engl. J. Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

