Antibody-Drug Conjugates for the Treatment of HER2-Positive Breast Cancer
- PMID: 36360302
- PMCID: PMC9691220
- DOI: 10.3390/genes13112065
Antibody-Drug Conjugates for the Treatment of HER2-Positive Breast Cancer
Abstract
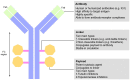
Human epidermal growth factor receptor 2 (HER2) receptor tyrosine kinase is overexpressed in 20-30% of breast cancers and is associated with poor prognosis and worse overall patient survival. Most women with HER2-positive breast cancer receive neoadjuvant chemotherapy plus HER2-targeted therapies. The development of HER2-directed therapeutics is an important advancement in targeting invasive breast cancer. Despite the efficacy of anti-HER2 monoclonal antibodies, they are still being combined with adjuvant chemotherapy to improve overall patient outcomes. Recently, significant progress has been made towards the development of a class of therapeutics known as antibody-drug conjugates (ADCs), which leverage the high specificity of HER2-targeted monoclonal antibodies with the potent cytotoxic effects of various small molecules, such as tubulin inhibitors and topoisomerase inhibitors. To date, two HER2-targeting ADCs have been approved by the FDA for the treatment of HER2-positive breast cancer: Ado-trastuzumab emtansine (T-DM1; Kadcyla®) and fam-trastuzumab deruxtecan-nxki (T-Dxd; Enhertu®). Kadcyla and Enhertu are approved for use as a second-line treatment after trastuzumab-taxane-based therapy in patients with HER2-positive breast cancer. The success of ADCs in the treatment of HER2-positive breast cancer provides novel therapeutic advancements in the management of the disease. In this review, we discuss the basic biology of HER2, its downstream signaling pathways, currently available anti-HER2 therapeutic modalities and their mechanisms of action, and the latest clinical and safety characteristics of ADCs used for the treatment of HER2-positive breast cancer.
Keywords: ADC; Enhertu; HER2; T-DM1; antibody drug conjugate; breast cancer; cancer; monoclonal antibody; therapeutics; trastuzumab.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cancello G., Maisonneuve P., Rotmensz N., Viale G., Mastropasqua M.G., Pruneri G., Montagna E., Iorfida M., Mazza M., Balduzzi A., et al. Progesterone receptor loss identifies Luminal B breast cancer subgroups at higher risk of relapse. Ann. Oncol. 2012;24:661–668. doi: 10.1093/annonc/mds430. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

