Real-World Outcomes of Systemic Therapy in Japanese Patients with Cancer (Tokushukai REAl-World Data Project: TREAD): Study Protocol for a Nationwide Cohort Study
- PMID: 36360487
- PMCID: PMC9690553
- DOI: 10.3390/healthcare10112146
Real-World Outcomes of Systemic Therapy in Japanese Patients with Cancer (Tokushukai REAl-World Data Project: TREAD): Study Protocol for a Nationwide Cohort Study
Abstract
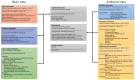
Cohort studies using large-scale databases have become increasingly important in recent years. The Tokushukai Medical Group is a leading medical group in Japan that includes 71 general hospitals nationwide from Hokkaido to Okinawa, with a total of 18,000 beds, and a unified electronic medical record system. This retrospective cohort study aims to evaluate the real-world outcomes of systemic therapy for Japanese patients with cancer using this merit of scale. All adult patients with cancer who received systemic therapy using a centrally registered chemotherapy protocol system at 46 hospitals from April 2010 to March 2020 will be identified (~48,850 patients). Key exclusion criteria include active double cancer and inadequate data extraction. Data will be obtained through electronic medical records, diagnosis procedure combination data, medical prescription data, and the national cancer registration system that includes sociodemographic variables, diagnostic and laboratory tests, concomitant drug prescriptions, cost, and overall survival. Kaplan-Meier estimates will be calculated for time-to-event analyses. Stratified/conventional Cox proportional hazards regression analyses will be conducted to examine the relationships between overall survival and related factors. Our findings provide important insights for future research directions, policy initiatives, medical guidelines, and clinical decision-making.
Keywords: National Cancer Registry; cancer chemotherapy; diagnosis procedure combination data; real-world data.
Conflict of interest statement
Some of the authors have received research funding, honoraria, or scholarship donations from various pharmaceutical companies and other organizations outside of the submitted work, none of which construe actual or potential conflicts of interest. Y.I. received speakers’ bureau fees/honoraria from Bayer, Bristol-Myers Squibb, Daiichi-Sankyo, Pfizer, and Ono Pharm, outside of the submitted work. H.M. has received speakers’ bureau fees/honoraria from Daiichi-Sankyo and Ono Pharm; research funding from Astelas-Amgen Biopharma, Bayer, Bristol Myers Squibb, Chugai, Daiichi-Sankyo, Incite, Novartis, Ono Pharm, Pfizer, and Rakuten-Medical; and scholarship donations from Bayer, Chugai, Daiichi-Sankyo, Eisai, Kyowa-Kirin, Lilly, Ono Pharmaceutical, Pfizer, Taiho Pharma, and Takeda, outside of the submitted work. These organizations had no role in the design, conduct, or reporting of this work.
Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Effect of adjuvant chemotherapy on survival benefit in stage III colon cancer patients stratified by age: a Japanese real-world cohort study.BMC Cancer. 2020 Jan 6;20(1):19. doi: 10.1186/s12885-019-6508-1. BMC Cancer. 2020. PMID: 31906959 Free PMC article.
-
Safety and Efficacy of Imatinib for Hospitalized Adults with COVID-19: A structured summary of a study protocol for a randomised controlled trial.Trials. 2020 Oct 28;21(1):897. doi: 10.1186/s13063-020-04819-9. Trials. 2020. PMID: 33115543 Free PMC article.
-
Real-World Survival Outcomes Associated With First-Line Immunotherapy, Targeted Therapy, and Combination Therapy for Metastatic Clear Cell Renal Cell Carcinoma.JAMA Netw Open. 2021 May 3;4(5):e2111329. doi: 10.1001/jamanetworkopen.2021.11329. JAMA Netw Open. 2021. PMID: 34032854 Free PMC article.
-
Toward the realization of a better aged society: messages from gerontology and geriatrics.Geriatr Gerontol Int. 2012 Jan;12(1):16-22. doi: 10.1111/j.1447-0594.2011.00776.x. Geriatr Gerontol Int. 2012. PMID: 22188494 Review.
Cited by
-
A cross domain access control model for medical consortium based on DBSCAN and penalty function.BMC Med Inform Decis Mak. 2024 Sep 16;24(1):260. doi: 10.1186/s12911-024-02638-5. BMC Med Inform Decis Mak. 2024. PMID: 39285411 Free PMC article.
-
Inflammation‑based prognostic markers in patients with advanced or recurrent gastric cancer treated with nivolumab: Tokushukai REAl‑world Data project 02 (TREAD 02).Mol Clin Oncol. 2024 Oct 3;21(6):90. doi: 10.3892/mco.2024.2788. eCollection 2024 Dec. Mol Clin Oncol. 2024. PMID: 39421231 Free PMC article.
-
Machine learning-based prediction of bleeding risk in extracorporeal membrane oxygenation patients using transfusion as a surrogate marker.Transfusion. 2025 Jun;65(6):1051-1060. doi: 10.1111/trf.18261. Epub 2025 Apr 25. Transfusion. 2025. PMID: 40277243 Free PMC article.
-
Analysis of aPTT predictors after unfractionated heparin administration in intensive care units using machine learning models.PLoS One. 2025 Jul 21;20(7):e0328709. doi: 10.1371/journal.pone.0328709. eCollection 2025. PLoS One. 2025. PMID: 40690448 Free PMC article.
-
Prognostic impact of concomitant pH-regulating drugs in patients with non-small cell lung cancer receiving epidermal growth factor receptor tyrosine kinase inhibitors: the Tokushukai REAl-world Data project 01-S1.Cancer Chemother Pharmacol. 2024 Aug;94(2):197-208. doi: 10.1007/s00280-024-04666-4. Epub 2024 Apr 8. Cancer Chemother Pharmacol. 2024. PMID: 38584202
References
LinkOut - more resources
Full Text Sources
Miscellaneous