Viral Response among Early Treated HIV Perinatally Infected Infants: Description of a Cohort in Southern Mozambique
- PMID: 36360495
- PMCID: PMC9691232
- DOI: 10.3390/healthcare10112156
Viral Response among Early Treated HIV Perinatally Infected Infants: Description of a Cohort in Southern Mozambique
Abstract
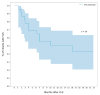
Early initiation of antiretroviral therapy and adherence to achieve viral load suppression (VLS) are crucial for reducing morbidity and mortality of perinatally HIV-infected infants. In this descriptive cohort study of 39 HIV perinatally infected infants, who started treatment at one month of life in Mozambique, we aimed to describe the viral response over 2 years of follow up. VLS ≤ 400 copies/mL, sustained VLS and viral rebound were described using a Kaplan-Meier estimator. Antiretroviral drug transmitted resistance was assessed for a sub-group of non-VLS infants. In total, 61% of infants reached VLS, and 50% had a rebound. Cumulative probability of VLS was 36%, 51%, and 69% at 6, 12 and 24 months of treatment, respectively. The median duration of VLS was 7.4 months (IQR 12.6) and the cumulative probability of rebound at 6 months was 30%. Two infants had resistance biomarkers to drugs included in their treatment regimen. Our findings point to a low rate of VLS and high rate of viral rebound. More frequent viral response monitoring is advisable to identify infants with rebound and offer timely adherence support. It is urgent to tailor the psychosocial support model of care to this specific age group and offer differentiated service delivery to mother-baby pairs.
Keywords: adherence; drug resistance; early antiretroviral therapy; pediatric HIV; viral load suppression; viral rebound.
Conflict of interest statement
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
-
- UNAIDS . Countdown to Zero: Global Plan towards the Elimination of New HIV Infections among Children by 2015 and Keeping Their Mothers Alive, 2011–2015. UNAIDS; Geneva, Switzerland: 2011.
-
- UNAIDS Start Free Stay Free AIDS Free—2019 report [Internet] 2019. [(accessed on 17 September 2019)]. Available online: https://www.unaids.org/en/resources/documents/2019/20190722_UNAIDS_SFSFA....
-
- UNAIDS Data 2021 [Internet] [(accessed on 5 June 2022)]. Available online: https://www.unaids.org/en/resources/documents/2021/2021_unaids_data.
-
- Bianchi F., Cohn J., Sacks E., Bailey R., Lemaire J.-F., Machekano R., Nzima V.N., Ekouévi P.F., Makone M., Odhiambo C.O., et al. Evaluation of a routine point-of-care intervention for early infant diagnosis of HIV: An observational study in eight African countries. Lancet HIV. 2019;6:e373–e381. doi: 10.1016/S2352-3018(19)30033-5. - DOI - PubMed
-
- Jani I.V., Meggi B., Loquiha O., Tobaiwa O., Mudenyanga C., Zitha A., Dadirayi M., Nedio M., Adolfo V., Timothy B., et al. Effect of point-of-care early infant diagnosis on antiretroviral therapy initiation and retention of patients. AIDS. 2018;32:1453. doi: 10.1097/QAD.0000000000001846. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

