Current Expertise, Opinions, and Attitude toward TNF-⍺ Antagonist Biosimilars among Physicians: A Self-Administered Online Survey in Western Switzerland
- PMID: 36360497
- PMCID: PMC9690245
- DOI: 10.3390/healthcare10112152
Current Expertise, Opinions, and Attitude toward TNF-⍺ Antagonist Biosimilars among Physicians: A Self-Administered Online Survey in Western Switzerland
Abstract
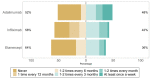
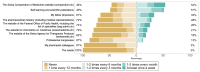
Tumor necrosis factor-alpha (TNF-⍺) antagonists are biological drugs with multiple authorized biosimilars. Biosimilars are becoming critical to the financial sustainability of health systems. Recent studies emphasize that physicians' knowledge regarding biosimilars has not yet progressed sufficiently to overcome their concerns regarding biosimilars' safety and efficacy. To assess the current knowledge, opinions, and attitudes toward TNF-⍺ antagonist biosimilars among postgraduate physicians and specialists, an anonymous, self-administered survey was implemented on SurveyMonkey between February and May 2022. The survey was validated through think-aloud interviews with senior and postgraduate physicians in rheumatology, gastroenterology, and immunoallergology, and a senior epidemiologist. Participant recruitment was conducted with the help of the physicians' professional societies and departmental head physicians of two university hospitals in Western Switzerland. Most physicians felt more comfortable initiating a TNF-⍺ antagonist biosimilar in biologic-naive patients (BNPs) than switching patients stabilized on the original biologic (originator). However, most participants agreed that BNPs should start treatment with the biosimilar rather than the originator when available. Postgraduate physicians and specialists in rheumatology, gastroenterology, and immunoallergology who participated in this survey were familiar with TNF-⍺ antagonist biosimilars and were confident in prescribing them. Yet, they still preferred to avoid switching a patient already on the originator.
Keywords: biosimilar adoption and commercialization; cost savings; physician incentive plans; tumor necrosing factor-alpha/tnf-alpha/survey.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Physicians' perceptions of the uptake of biosimilars: a systematic review.BMJ Open. 2020 May 5;10(5):e034183. doi: 10.1136/bmjopen-2019-034183. BMJ Open. 2020. PMID: 32371511 Free PMC article.
-
Physicians' and patients' perception of biosimilars and factors affecting biosimilar prescribing in selected Asian countries: a survey study.Expert Opin Biol Ther. 2024 Oct;24(10):1171-1182. doi: 10.1080/14712598.2024.2400523. Epub 2024 Sep 17. Expert Opin Biol Ther. 2024. PMID: 39234698
-
Medical specialists' attitudes to prescribing biosimilars.Pharmacoepidemiol Drug Saf. 2017 May;26(5):570-577. doi: 10.1002/pds.4186. Epub 2017 Feb 24. Pharmacoepidemiol Drug Saf. 2017. PMID: 28233367
-
Practical Management of Biosimilar Use in Inflammatory Bowel Disease (IBD): A Global Survey and an International Delphi Consensus.J Clin Med. 2023 Oct 3;12(19):6350. doi: 10.3390/jcm12196350. J Clin Med. 2023. PMID: 37834994 Free PMC article.
-
Pharmacists' Perspectives of Biosimilars: A Systematic Review.BioDrugs. 2022 Jul;36(4):489-508. doi: 10.1007/s40259-022-00541-x. Epub 2022 Jul 1. BioDrugs. 2022. PMID: 35776294
Cited by
-
Barriers and Enablers Affecting the Uptake of Biosimilar Medicines Viewed Through the Lens of Actor Network Theory: A Systematic Review.BioDrugs. 2024 Jul;38(4):541-555. doi: 10.1007/s40259-024-00659-0. Epub 2024 Jun 15. BioDrugs. 2024. PMID: 38879730 Free PMC article.
References
-
- BCC Research Biologic Therapeutic Drugs: Technologies and Global Markets. [(accessed on 14 September 2021)]. Available online: https://www.bccresearch.com/market-research/biotechnology/biologic-thera....
-
- Mikulic M. Global Pharmaceutical Industry–Statistics & Facts. [(accessed on 14 September 2021)]. Available online: https://www.statista.com/topics/1764/global-pharmaceutical-industry/
-
- Institute for Human Data Science (IQVIA) The Impact of Biosimilar Competition in Europe. [(accessed on 16 March 2022)]. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/the-global-us....
-
- Institute for Human Data Science (IQVIA) Advancing Biosimilar Sustainability in Europe: A Multi-Stakeholder Assessment. [(accessed on 24 February 2020)]. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/advancing-bio....
-
- Institute fo Human Data Science (IQVIA) The Global Use of Medicine in 2019 and Outlook to 2023. [(accessed on 6 February 2020)]. Available online: https://www.iqvia.com/-/media/iqvia/pdfs/institute-reports/the-global-us....
LinkOut - more resources
Full Text Sources

