Oral Microcosm Biofilms Grown under Conditions Progressing from Peri-Implant Health, Peri-Implant Mucositis, and Peri-Implantitis
- PMID: 36360970
- PMCID: PMC9654334
- DOI: 10.3390/ijerph192114088
Oral Microcosm Biofilms Grown under Conditions Progressing from Peri-Implant Health, Peri-Implant Mucositis, and Peri-Implantitis
Abstract
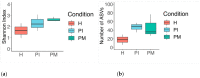
Peri-implantitis is a disease influenced by dysbiotic microbial communities that play a role in the short- and long-term outcomes of its clinical treatment. The ecological triggers that establish the progression from peri-implant mucositis to peri-implantitis remain unknown. This investigation describes the development of a novel in vitro microcosm biofilm model. Biofilms were grown over 30 days over machined titanium discs in a constant depth film fermentor (CDFF), which was inoculated (I) with pooled human saliva. Following longitudinal biofilm sampling across peri-implant health (PH), peri-implant mucositis (PM), and peri-implantitis (PI) conditions, the characterisation of the biofilms was performed. The biofilm analyses included imaging by confocal laser scanning microscopy (CLSM) and scanning electron microscopy (SEM), selective and non-selective culture media of viable biofilms, and 16S rRNA gene amplification and sequencing. Bacterial qualitative shifts were observed by CLSM and SEM across conditions, which were defined by characteristic phenotypes. A total of 9 phyla, 83 genera, and 156 species were identified throughout the experiment. The phyla Proteobacteria, Bacteroidetes, Firmicutes, Fusobacteria, and Actinobacteria showed the highest prevalence in PI conditions. This novel in vitro microcosm model provides a high-throughput alternative for growing microcosm biofilms resembling an in vitro progression from PH-PM-PI conditions.
Keywords: microbial ecology; microbiome; microcosm; modelling biofilms; oral biofilm; peri-implantitis; therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Berglundh T., Armitage G., Araujo M.G., Avila-Ortiz G., Blanco J., Camargo P.M., Chen S., Cochran D., Derks J., Figuero E., et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018;45((Suppl. 20)):S286–S291. doi: 10.1111/jcpe.12957. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

