Dual-Functionalized Nanoliposomes Achieve a Synergistic Chemo-Phototherapeutic Effect
- PMID: 36361615
- PMCID: PMC9653560
- DOI: 10.3390/ijms232112817
Dual-Functionalized Nanoliposomes Achieve a Synergistic Chemo-Phototherapeutic Effect
Abstract
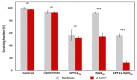
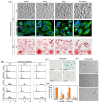
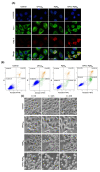
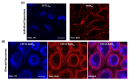
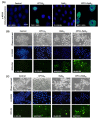
The enhancement of photodynamic therapy (PDT) effectiveness by combining it with other treatment modalities and improved drug delivery has become an interesting field in cancer research. We have prepared and characterized nanoliposomes containing the chemotherapeutic drug irinotecan (CPT11lip), the photodynamic agent protoporphyrin IX (PpIXlip), or their combination (CPT11-PpIXlip). The effects of individual and bimodal (chemo-phototherapeutic) treatments on HeLa cells have been studied by a combination of biological and photophysical studies. Bimodal treatments show synergistic cytotoxic effects on HeLa cells at relatively low doses of PpIX/PDT and CPT11. Mechanistic cell inactivation studies revealed mitotic catastrophe, apoptosis, and senescence contributions. The enhanced anticancer activity is due to a sustained generation of reactive oxygen species, which increases the number of double-strand DNA breaks. Bimodal chemo-phototherapeutic liposomes may have a very promising future in oncological therapy, potentially allowing a reduction in the CPT11 concentration required to achieve a therapeutic effect and overcoming resistance to individual cancer treatments.
Keywords: bimodal-functionalized nanoliposomes; chemo-phototherapy; double-strand DNA break; irinotecan; photodynamic therapy; protoporphyrin IX; reactive oxygen species; subcellular location; synergistic effect; time-lapse microscopy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

