Phosphorylated Tau in Alzheimer's Disease and Other Tauopathies
- PMID: 36361631
- PMCID: PMC9654278
- DOI: 10.3390/ijms232112841
Phosphorylated Tau in Alzheimer's Disease and Other Tauopathies
Abstract
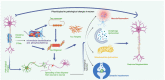
Alzheimer's disease (AD) is the leading cause of dementia in elderly people. Amyloid beta (Aβ) deposits and neurofibrillary tangles are the major pathological features in an Alzheimer's brain. These proteins are highly expressed in nerve cells and found in most tissues. Tau primarily provides stabilization to microtubules in the part of axons and dendrites. However, tau in a pathological state becomes hyperphosphorylated, causing tau dysfunction and leading to synaptic impairment and degeneration of neurons. This article presents a summary of the role of tau, phosphorylated tau (p-tau) in AD, and other tauopathies. Tauopathies, including Pick's disease, frontotemporal dementia, corticobasal degeneration, Alzheimer's disease, argyrophilic grain disease, progressive supranuclear palsy, and Huntington's disease, are the result of misprocessing and accumulation of tau within the neuronal and glial cells. This article also focuses on current research on the post-translational modifications and genetics of tau, tau pathology, the role of tau in tauopathies and the development of new drugs targeting p-tau, and the therapeutics for treating and possibly preventing tauopathies.
Keywords: Alzheimer’s disease; amyloid beta; dementia; oxidative stress; phosphorylated tau; tauopathy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Morton H., Kshirsagar S., Orlov E., Bunquin L.E., Sawant N., Boleng L., George M., Basu T., Ramasubramanian B., Pradeepkiran J.A. Defective mitophagy and synaptic degeneration in Alzheimer’s disease: Focus on aging, mitochondria and synapse. Free Radic. Biol. Med. 2021;172:652–667. doi: 10.1016/j.freeradbiomed.2021.07.013. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

