NLRP3-Mediated Piezo1 Upregulation in ACC Inhibitory Parvalbumin-Expressing Interneurons Is Involved in Pain Processing after Peripheral Nerve Injury
- PMID: 36361825
- PMCID: PMC9655876
- DOI: 10.3390/ijms232113035
NLRP3-Mediated Piezo1 Upregulation in ACC Inhibitory Parvalbumin-Expressing Interneurons Is Involved in Pain Processing after Peripheral Nerve Injury
Abstract
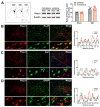
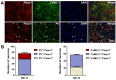
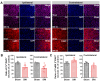
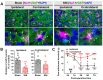
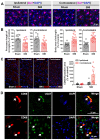
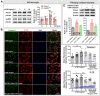
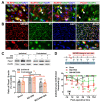
The anterior cingulate cortex (ACC) is particularly critical for pain information processing. Peripheral nerve injury triggers neuronal hyper-excitability in the ACC and mediates descending facilitation to the spinal dorsal horn. The mechanically gated ion channel Piezo1 is involved in the transmission of pain information in the peripheral nervous system. However, the pain-processing role of Piezo1 in the brain is unknown. In this work, we found that spared (sciatic) nerve injury (SNI) increased Piezo1 protein levels in inhibitory parvalbumin (PV)-expressing interneurons (PV-INs) but not in glutaminergic CaMKⅡ+ neurons, in the bilateral ACC. A reduction in the number of PV-INs but not in the number of CaMKⅡ+ neurons and a significant reduction in inhibitory synaptic terminals was observed in the SNI chronic pain model. Further, observation of morphological changes in the microglia in the ACC showed their activated amoeba-like transformation, with a reduction in process length and an increase in cell body area. Combined with the encapsulation of Piezo1-positive neurons by Iba1+ microglia, the loss of PV-INs after SNI might result from phagocytosis by the microglia. In cellular experiments, administration of recombinant rat TNF-α (rrTNF) to the BV2 cell culture or ACC neuron primary culture elevated the protein levels of Piezo1 and NOD-like receptor (NLR) family pyrin domain containing 3 (NLRP3). The administration of the NLRP3 inhibitor MCC950 in these cells blocked the rrTNF-induced expression of caspase-1 and interleukin-1β (key downstream factors of the activated NLRP3 inflammasome) in vitro and reversed the SNI-induced Piezo1 overexpression in the ACC and alleviated SNI-induced allodynia in vivo. These results suggest that NLRP3 may be the key factor in causing Piezo1 upregulation in SNI, promoting an imbalance between ACC excitation and inhibition by inducing the microglial phagocytosis of PV-INs and, thereby, facilitating spinal pain transmission.
Keywords: NLRP3; Piezo1; anterior cingulate cortex; neuropathic pain; parvalbumin interneuron.
Conflict of interest statement
The authors declare no conflict to interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

