Granulocytic MDSC with Deficient CCR5 Alleviates Lipogenesis and Inflammation in Nonalcoholic Fatty Liver Disease
- PMID: 36361830
- PMCID: PMC9656569
- DOI: 10.3390/ijms232113048
Granulocytic MDSC with Deficient CCR5 Alleviates Lipogenesis and Inflammation in Nonalcoholic Fatty Liver Disease
Abstract
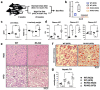
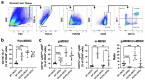
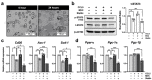
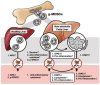
C-C chemokine receptor type 5 (CCR5) positively contributes to the pathogenesis of nonalcoholic fatty liver disease (NAFLD), a common metabolic liver disease associated with chronic inflammation. CCR5 signaling also facilitates the immunosuppressive activity of a group of immature myeloid cells known as granulocytic myeloid-derived suppressor cells (g-MDSCs). While both hepatocyte and g-MDSC express CCR5, how CCR5 coordinates these two distinct cell types in the hepatic microenvironment remains largely unknown. Here, we used in vivo and ex vivo approaches to define the molecular details of how CCR5 mediates the crosstalk between hepatocytes and g-MDSCs in a mouse model of NAFLD. Global CCR5-deficient mice exhibited more severe steatosis, increased hepatic gene expression of lipogenesis, and exacerbated liver damage in diet-induced obesity. Either NAFLD or CCR5-deficiency per se is causative for the increase of g-MDSCs. Purified g-MDSCs have a higher survival rate in the fatty liver microenvironment, and blockade of CCR5 significantly decreases g-MDSCs' expression of anti-inflammatory factors. On the other hand, the null of CCR5 signaling increases hepatocytes' expression of lipogenic genes in the NAFLD microenvironment. Most importantly, inhibiting g-MDSCs' CCR5 signaling in the fatty liver microenvironment dramatically reduces STAT3 signaling, lipogenic, and pro-inflammatory gene expression in primary hepatocytes. Adoptive cell transfer experiments further demonstrate that CCR5-deficient g-MDSCs mitigate hepatic lipogenic gene expression without facilitating pro-inflammatory cytokine production and liver damage in NAFLD mice. These results suggest that targeting g-MDSCs' CCR5 signaling might serve as a potential therapeutic strategy for NAFLD.
Keywords: CCR5; NAFLD; STAT3; g-MDSC; inflammation; lipid metabolism.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures




References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

