Dual Inhibitors of AChE and BACE-1 for Reducing Aβ in Alzheimer's Disease: From In Silico to In Vivo
- PMID: 36361906
- PMCID: PMC9655245
- DOI: 10.3390/ijms232113098
Dual Inhibitors of AChE and BACE-1 for Reducing Aβ in Alzheimer's Disease: From In Silico to In Vivo
Abstract
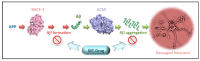
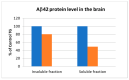
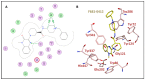
Alzheimer's disease (AD) is a complex and widespread condition, still not fully understood and with no cure yet. Amyloid beta (Aβ) peptide is suspected to be a major cause of AD, and therefore, simultaneously blocking its formation and aggregation by inhibition of the enzymes BACE-1 (β-secretase) and AChE (acetylcholinesterase) by a single inhibitor may be an effective therapeutic approach, as compared to blocking one of these targets or by combining two drugs, one for each of these targets. We used our ISE algorithm to model each of the AChE peripheral site inhibitors and BACE-1 inhibitors, on the basis of published data, and constructed classification models for each. Subsequently, we screened large molecular databases with both models. Top scored molecules were docked into AChE and BACE-1 crystal structures, and 36 Molecules with the best weighted scores (based on ISE indexes and docking results) were sent for inhibition studies on the two enzymes. Two of them inhibited both AChE (IC50 between 4-7 μM) and BACE-1 (IC50 between 50-65 μM). Two additional molecules inhibited only AChE, and another two molecules inhibited only BACE-1. Preliminary testing of inhibition by F681-0222 (molecule 2) on APPswe/PS1dE9 transgenic mice shows a reduction in brain tissue of soluble Aβ42.
Keywords: acetylcholinesterase (AChE); amyloid beta; dual inhibitors; enzyme inhibition; in silico; in vitro; in vivo; multi-targeting; β-secretase (BACE-1).
Conflict of interest statement
All authors declare no conflict of interest.
Figures




References
-
- [(accessed on 31 January 2022)]; Available online: http://www.nia.nih.gov/alzheimers/topics/symptoms.
-
- Plascencia-Villa G., Perry G. Status and future directions of clinical trials in Alzheimer’s disease. Int. Rev. Neurobiol. 2020;154:3–50. - PubMed
-
- Savelieff M.G., Nam G., Kang J., Lee H.J., Lee M., Lim M.H. Development of Multifunctional Molecules as Potential Therapeutic Candidates for Alzheimer’s Disease, Parkinson’s Disease, and Amyotrophic Lateral Sclerosis in the Last Decade. Chem. Rev. 2019;119:1221–1322. doi: 10.1021/acs.chemrev.8b00138. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

