Benzoylpaeoniflorin Activates Anti-Inflammatory Mechanisms to Mitigate Sepsis in Cell-Culture and Mouse Sepsis Models
- PMID: 36361915
- PMCID: PMC9656632
- DOI: 10.3390/ijms232113130
Benzoylpaeoniflorin Activates Anti-Inflammatory Mechanisms to Mitigate Sepsis in Cell-Culture and Mouse Sepsis Models
Abstract
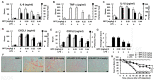
Xuebijing injection (XBJI) (comprising of five herbs) is a widely used traditional Chinese medicine for sepsis treatment. However, the bioactive components of XBJI and the mechanisms responsible for its sepsis-mitigating action have not been experimentally determined. One of the main bioactive compounds in XBJI-benzoylpaeoniflorin (BPF)-inhibits the expressions of key mediators of inflammation such as nuclear factor kappa B (NF-κB), cyclooxygenase-1 (COX-1), and COX-2. However, its effects on sepsis have not been determined yet. Therefore, here, we investigated the immunomodulatory effect of BPF on severely inflamed endothelial cells, THP-1 macrophages, peritoneal macrophages, and mice. Human umbilical vein endothelial cells (HUVECs) and THP-1-macrophages were activated using lipopolysaccharide (LPS) after pretreatment with BPF. Subsequently, changes in the expression profiles of pro-inflammatory molecules including inducible nitric oxide synthase (iNOS), tumor necrosis factor (TNF)-α, and interleukin (IL)-6 were determined using quantitative real-time polymerase chain reaction (qPCR) and Western blot analysis. Furthermore, we monitored the phosphorylation of NF-kB and mitogen-activated protein kinases (MAPKs) to determine their activation levels. Using the LPS-induced mouse model of sepsis, we studied the effects of BPF on inflammatory cytokine production, pulmonary histopathology, and survival rates. Finally, we evaluated whether BPF protects against cecal ligation and puncture (CLP)-induced sepsis, as it closely mimics human sepsis. BPF pretreatment inhibited LPS-induced increase in mRNA and protein levels of iNOS, TNF-α, and IL-6 in HUVECs and THP-1-macrophages. It also suppressed LPS-mediated phosphorylation of p65, p38, JNK, and ERK. Mice with LPS-induced-sepsis who were treated with BPF had lower serum levels of IL-6, TNF-α, IL-1β, CXCL1, and CXCL2 than the control mice treated with BPF. Histopathology revealed that BPF treatment alleviated LPS-induced lung damage. In addition, in mice given a lethal dose of LPS, BPF treatment showed a dose-dependent improvement in survival rates. BPF treatment dose-dependently inhibited the LPS-induced IL-6, TNF-α, and CXCL1 production in peritoneal macrophages. BPF treatment also dose-dependently improved the survival rates in mice with CLP-induced sepsis. These results show that BPF alleviates LPS-stimulated septic conditions and protects mice from CLP-induced sepsis. Our research marks BPF as a potential drug in the treatment of sepsis and various inflammatory diseases.
Keywords: CLP; benzoylpaeoniflorin; endothelium; inflammation; lipopolysaccharide; sepsis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
MeSH terms
Substances
Grants and funding
- HI15C0001/Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea
- 2020R1A2C1004131 and 2022R1A4A10189001/National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

