Monitoring the Simultaneous Implantation of Ti and Tb Cations to a Sacrificial Template and the Sol-Gel Synthesis of Tb-Doped TiO2 (Anatase) Hollow Spheres and Their Transition to Rutile Phase
- PMID: 36361949
- PMCID: PMC9657842
- DOI: 10.3390/ijms232113162
Monitoring the Simultaneous Implantation of Ti and Tb Cations to a Sacrificial Template and the Sol-Gel Synthesis of Tb-Doped TiO2 (Anatase) Hollow Spheres and Their Transition to Rutile Phase
Abstract
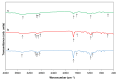
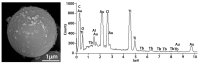
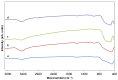
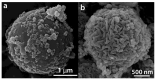
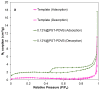
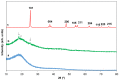
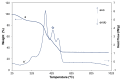
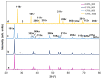
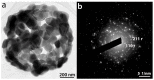
Tb-doped TiO2 (anatase) micro-hollow spheres (HSs) with nano-shells, in the range 0.00-3.00 at.% Tb, were successfully synthesized by a simultaneous chemical implantation route of both Ti and Tb cations from chlorides to a poly-styrene (PST)-co-poly-divinyl benzene (PDVB) sacrificial template, followed by controlled hydrolysis and polycondensation reactions. After water addition to the mixture of the precursors with the template, a decrease in the intensity and a shift to lower wavenumbers of the C=O absorption band in the IR spectra can indicate not only the anchoring of Ti and Tb ions to the carbonyl group of the template but also the hydrolysis of the implanted precursors. This latter process can involve a proton attack on the Ti-Cl, Tb-Cl and C=O bonds, the occupation of a vacant site by a water molecule, and then the dissociation of the dangling Ti-Cl, Tb-Cl ligands and C=O bonds. It gives rise to Ti1-xTbx[(OH)4-uClv]@PST-PDVB and Ti1-xTbx[(OH)4-y]@PST-PDVB complexes (x = 0.00, 0.0012, 0.0170 and 0.030). Finally, polycondensation of these species leads to Ti1-xTbxO2-w'@PST-PDVB compounds. After subsequent thermal removal at 550 °C of the template, the IR bands of the core (template) totally vanished and new bands were observed in the 400-900 cm-1 region which can be attributed to the metalloxane bondings (M-O, M'-O, M-O-M, M-O-M' and/or M'-O-M', being M and M' = Ti and Tb, respectively, i.e., mainly vibration modes of anatase). Then, micron-sized HSs of TiO2 and Tb-doped-TiO2 (anatase) were obtained with nano-shells according to field emission gun scanning electron microscopy (FEG-SEM) and transmission electron microscopy (TEM) observations. Furthermore, X-ray photoelectron spectroscopy (XPS) measurements confirmed the presence of Tb4+ (38.5 and 41.2% for 1.70 and 3.00 at.% Tb, respectively) in addition to Tb3+ in the resulting HSs, with increasing Tb4+ content with both Tb doping and higher calcination temperatures. Then, these HSs can be considered as rare earth (RE) co-doped systems, at least for 1.70 and 3.00 at.% Tb contents being the transition to rutile phase favored by Tb doping for those compositions. Finally, diffusion of Tb from the inner parts to the surface of the HSs with the calcination treatments was also observed by XPS.
Keywords: Tb; TiO2; X-ray methods; hollow sphere; implantation; sol-gel.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Bjelajac A., Petrovic R., Socol G., Mihailescu V., Grumezescu V., Pavlovic V. CdS quantum dots sensitized TiO2 nanotubes by matrix assisted pulsed laser evaporation method. Ceram. Intern. 2016;42:9011–9017. doi: 10.1016/j.ceramint.2016.02.159. - DOI
-
- Marien C., Cottineau T., Robert D. TiO2 Nanotube arrays: Influence of tube length on the photocatalytic degradation of paraquat. Appl. Catal. B. 2016;194:1–6. doi: 10.1016/j.apcatb.2016.04.040. - DOI
-
- Borlaf M., Wu H.-M., Colomer M.T., Moreno R., Tseng W.J. Synthesis and characterization of anatase-structured titania hollow spheres doped with erbium (III) J. Am. Ceram. Soc. 2012;95:3005–3011. doi: 10.1111/j.1551-2916.2012.05329.x. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

