Blockage of KHSRP-NLRP3 by MCC950 Can Reverse the Effect of Manganese-Induced Neuroinflammation in N2a Cells and Rat Brain
- PMID: 36362011
- PMCID: PMC9658363
- DOI: 10.3390/ijms232113224
Blockage of KHSRP-NLRP3 by MCC950 Can Reverse the Effect of Manganese-Induced Neuroinflammation in N2a Cells and Rat Brain
Abstract
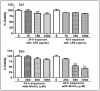
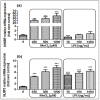
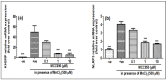
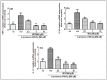
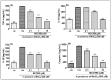
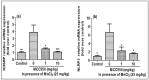
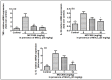
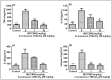
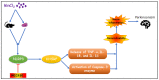
Manganese neurotoxicity has been reported to cause a neurodegenerative disease known as parkinsonism. Previous reports have shown that the expression of the KH-type splicing regulatory protein (KHSRP), a nucleic acid-binding protein, and NLRP3 is increased upon Mn exposure. However, the relation between these two during Mn toxicity has not been fully deduced. The mouse neuroblastoma (N2a) and SD rats are treated with LPS and MnCl2 to evaluate the expression of KHSRP and NLRP3. Further, the effect of the NLRP3 inhibitor MCC950 is checked on the expression of NLRP3, KHSRP and pro-inflammatory markers (TNFα, IL-18 and IL-1β) as well as the caspase-1 enzyme. Our results demonstrated an increment in NLRP3 and KHSRP expression post-MnCl2 exposure in N2a cells and rat brain, while on the other hand with LPS exposure only NLRP3 expression levels were elevated and KHSRP was found to be unaffected. An increased expression of KHSRP, NLRP3, pro-inflammatory markers and the caspase-1 enzyme was observed to be inhibited with MCC950 treatment in MnCl2-exposed cells and rats. Manganese exposure induces NLRP3 and KHSRP expression to induce neuroinflammation, suggesting a correlation between both which functions in toxicity-related pathways. Furthermore, MCC950 treatment reversed the role of KHSRP from anti-inflammatory to pro-inflammatory.
Keywords: KHSRP; N2a cells; Parkinson’s; manganese neurotoxicity; neuroinflammation.
Conflict of interest statement
All the authors declare no conflict of interest.
Figures



References
-
- Rybakowska I., Kaletha K., Anand J.S. Manganism–Neurodegenerative Brain Disease Caused by Poisoning with Manganese. Prz. Lek. 2012;69:555–556. - PubMed
-
- Ávila D.S., Puntel R.L., Folmer V., Rocha J.B.T., dos Santos A.P.M., Aschner M. Manganese Neurotoxicity. In: Kostrzewa R.M., editor. Handbook of Neurotoxicity. Springer; New York, NY, USA: 2014. pp. 843–864.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

