Recent Advances in Endocannabinoid System Targeting for Improved Specificity: Strategic Approaches to Targeted Drug Delivery
- PMID: 36362014
- PMCID: PMC9658826
- DOI: 10.3390/ijms232113223
Recent Advances in Endocannabinoid System Targeting for Improved Specificity: Strategic Approaches to Targeted Drug Delivery
Abstract
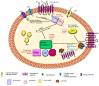
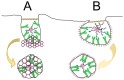
Opportunities for developing innovative and intelligent drug delivery technologies by targeting the endocannabinoid system are becoming more apparent. This review provides an overview of strategies to develop targeted drug delivery using the endocannabinoid system (ECS). Recent advances in endocannabinoid system targeting showcase enhanced pharmaceutical therapy specificity while minimizing undesirable side effects and overcoming formulation challenges associated with cannabinoids. This review identifies advances in targeted drug delivery technologies that may permit access to the full pharmacotherapeutic potential of the ECS. The design of optimized nanocarriers that target specific tissues can be improved by understanding the nature of the signaling pathways, distribution in the mammalian body, receptor structure, and enzymatic degradation of the ECS. A closer look at ligand-receptor complexes, endocannabinoid tone, tissue distribution, and G-protein activity leads to a better understanding of the potential of the ECS toolkit for therapeutics. The signal transduction pathways examine the modulation of downstream effector proteins, desensitization, signaling cascades, and biased signaling. An in-depth and overall view of the targeted system is achieved through homology modeling where mutagenesis and ligand binding examine the binding site and allow sequence analysis and the formation of libraries for molecular docking and molecular dynamic simulations. Internalization routes exploring receptor-mediated endocytosis and lipid rafts are also considered for explicit signaling. Furthermore, the review highlights nanotechnology and surface modification aspects as a possible future approach for specific targeting.
Keywords: allosteric modulation; biased signaling; endocannabinoid system; endocannabinoid tone; g-protein coupled receptors; receptor-mediated drug delivery; surface-modified nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


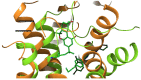

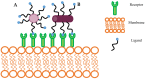
Similar articles
-
Lipid rafts: a nexus for endocannabinoid signaling?Life Sci. 2005 Aug 19;77(14):1640-50. doi: 10.1016/j.lfs.2005.05.010. Life Sci. 2005. PMID: 15978630 Review.
-
Cannabinoid pharmacology/therapeutics in chronic degenerative disorders affecting the central nervous system.Biochem Pharmacol. 2018 Nov;157:67-84. doi: 10.1016/j.bcp.2018.08.016. Epub 2018 Aug 17. Biochem Pharmacol. 2018. PMID: 30121249 Review.
-
Targeting the endocannabinoid system: future therapeutic strategies.Drug Discov Today. 2017 Jan;22(1):105-110. doi: 10.1016/j.drudis.2016.08.005. Epub 2016 Aug 20. Drug Discov Today. 2017. PMID: 27554802 Review.
-
Seeing over the horizon - targeting the endocannabinoid system for the treatment of ocular disease.J Basic Clin Physiol Pharmacol. 2016 May 1;27(3):253-65. doi: 10.1515/jbcpp-2015-0065. J Basic Clin Physiol Pharmacol. 2016. PMID: 26565550 Review.
-
Structural and Functional Insights into Cannabinoid Receptors.Trends Pharmacol Sci. 2020 Sep;41(9):665-677. doi: 10.1016/j.tips.2020.06.010. Epub 2020 Jul 29. Trends Pharmacol Sci. 2020. PMID: 32739033 Review.
Cited by
-
Cannabinoids: Potential for Modulation and Enhancement When Combined with Vitamin B12 in Case of Neurodegenerative Disorders.Pharmaceuticals (Basel). 2024 Jun 20;17(6):813. doi: 10.3390/ph17060813. Pharmaceuticals (Basel). 2024. PMID: 38931480 Free PMC article. Review.
-
Sleep, Glial Function, and the Endocannabinoid System: Implications for Neuroinflammation and Sleep Disorders.Int J Mol Sci. 2024 Mar 9;25(6):3160. doi: 10.3390/ijms25063160. Int J Mol Sci. 2024. PMID: 38542134 Free PMC article. Review.
-
Exploring the therapeutic potential of natural compounds modulating the endocannabinoid system in various diseases and disorders: review.Pharmacol Rep. 2023 Dec;75(6):1410-1444. doi: 10.1007/s43440-023-00544-7. Epub 2023 Oct 31. Pharmacol Rep. 2023. PMID: 37906390 Review.
-
Cannabinoid-Based Ocular Therapies and Formulations.Pharmaceutics. 2023 Mar 27;15(4):1077. doi: 10.3390/pharmaceutics15041077. Pharmaceutics. 2023. PMID: 37111563 Free PMC article. Review.
-
The endocannabinoid system in appetite regulation and treatment of obesity.Pharmacol Res Perspect. 2024 Oct;12(5):e70009. doi: 10.1002/prp2.70009. Pharmacol Res Perspect. 2024. PMID: 39292202 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

