AmyP53, a Therapeutic Peptide Candidate for the Treatment of Alzheimer's and Parkinson's Disease: Safety, Stability, Pharmacokinetics Parameters and Nose-to Brain Delivery
- PMID: 36362170
- PMCID: PMC9654333
- DOI: 10.3390/ijms232113383
AmyP53, a Therapeutic Peptide Candidate for the Treatment of Alzheimer's and Parkinson's Disease: Safety, Stability, Pharmacokinetics Parameters and Nose-to Brain Delivery
Abstract
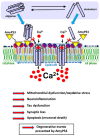
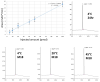
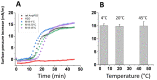
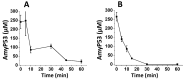
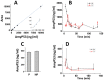
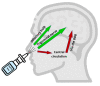
Neurodegenerative disorders are a major public health issue. Despite decades of research efforts, we are still seeking an efficient cure for these pathologies. The initial paradigm of large aggregates of amyloid proteins (amyloid plaques, Lewis bodies) as the root cause of Alzheimer's and Parkinson's diseases has been mostly dismissed. Instead, membrane-bound oligomers forming Ca2+-permeable amyloid pores are now considered appropriate targets for these diseases. Over the last 20 years, our group deciphered the molecular mechanisms of amyloid pore formation, which appeared to involve a common pathway for all amyloid proteins, including Aβ (Alzheimer) and α-synuclein (Parkinson). We then designed a short peptide (AmyP53), which prevents amyloid pore formation by targeting gangliosides, the plasma membrane receptors of amyloid proteins. Herein, we show that aqueous solutions of AmyP53 are remarkably stable upon storage at temperatures up to 45 °C for several months. AmyP53 appeared to be more stable in whole blood than in plasma. Pharmacokinetics studies in rats demonstrated that the peptide can rapidly and safely reach the brain after intranasal administration. The data suggest both the direct transport of AmyP53 via the olfactory bulb (and/or the trigeminal nerve) and an indirect transport via the circulation and the blood-brain barrier. In vitro experiments confirmed that AmyP53 is as active as cargo peptides in crossing the blood-brain barrier, consistent with its amino acid sequence specificities and physicochemical properties. Overall, these data open a route for the use of a nasal spray formulation of AmyP53 for the prevention and/or treatment of Alzheimer's and Parkinson's diseases in future clinical trials in humans.
Keywords: Alzheimer’s; Parkinson’s; amyloid pore; ganglioside; peptide; therapy.
Conflict of interest statement
N.Y. and J.F. are co-inventors of the AmyP53 peptide (patent Application EP15709163.8A), currently under development for the treatment of Alzheimer’s and Parkinson’s diseases by the AmyPore company. H.C. and C.D.S. are, respectively, the president and a member of the Ethics and Scientific Committee of AmyPore. All other authors do not have any conflict of interest.
Figures


Similar articles
-
AmyP53 Prevents the Formation of Neurotoxic β-Amyloid Oligomers through an Unprecedent Mechanism of Interaction with Gangliosides: Insights for Alzheimer's Disease Therapy.Int J Mol Sci. 2023 Jan 16;24(2):1760. doi: 10.3390/ijms24021760. Int J Mol Sci. 2023. PMID: 36675271 Free PMC article.
-
Gene Therapy Strategy for Alzheimer's and Parkinson's Diseases Aimed at Preventing the Formation of Neurotoxic Oligomers in SH-SY5Y Cells.Int J Mol Sci. 2021 Oct 26;22(21):11550. doi: 10.3390/ijms222111550. Int J Mol Sci. 2021. PMID: 34768981 Free PMC article.
-
Broad neutralization of calcium-permeable amyloid pore channels with a chimeric Alzheimer/Parkinson peptide targeting brain gangliosides.Biochim Biophys Acta. 2016 Feb;1862(2):213-22. doi: 10.1016/j.bbadis.2015.11.012. Epub 2015 Dec 2. Biochim Biophys Acta. 2016. PMID: 26655601
-
Insights into the Molecular Mechanisms of Alzheimer's and Parkinson's Diseases with Molecular Simulations: Understanding the Roles of Artificial and Pathological Missense Mutations in Intrinsically Disordered Proteins Related to Pathology.Int J Mol Sci. 2018 Jan 24;19(2):336. doi: 10.3390/ijms19020336. Int J Mol Sci. 2018. PMID: 29364151 Free PMC article. Review.
-
Progress toward Alzheimer's disease treatment: Leveraging the Achilles' heel of Aβ oligomers?Protein Sci. 2020 Aug;29(8):1748-1759. doi: 10.1002/pro.3906. Epub 2020 Jul 13. Protein Sci. 2020. PMID: 32567070 Free PMC article. Review.
Cited by
-
AmyP53 Prevents the Formation of Neurotoxic β-Amyloid Oligomers through an Unprecedent Mechanism of Interaction with Gangliosides: Insights for Alzheimer's Disease Therapy.Int J Mol Sci. 2023 Jan 16;24(2):1760. doi: 10.3390/ijms24021760. Int J Mol Sci. 2023. PMID: 36675271 Free PMC article.
-
Molecular Mechanism of Alzheimer's Disease.Int J Mol Sci. 2023 Nov 28;24(23):16837. doi: 10.3390/ijms242316837. Int J Mol Sci. 2023. PMID: 38069160 Free PMC article.
-
Interactions of alpha-synuclein with membranes in Parkinson's disease: Mechanisms and therapeutic strategies.Neurobiol Dis. 2024 Oct 15;201:106646. doi: 10.1016/j.nbd.2024.106646. Epub 2024 Aug 22. Neurobiol Dis. 2024. PMID: 39181187 Free PMC article. Review.
-
Overcoming the Blood-Brain Barrier for Drug Delivery to the Brain.ACS Omega. 2025 Jul 22;10(30):32544-32563. doi: 10.1021/acsomega.5c00364. eCollection 2025 Aug 5. ACS Omega. 2025. PMID: 40787406 Free PMC article. Review.
-
Lipid rafts and human diseases: why we need to target gangliosides.FEBS Open Bio. 2023 Sep;13(9):1636-1650. doi: 10.1002/2211-5463.13612. Epub 2023 Apr 20. FEBS Open Bio. 2023. PMID: 37052878 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

