METCAM/MUC18 Plays a Tumor Suppressor Role in the Development of Nasopharyngeal Carcinoma Type I
- PMID: 36362174
- PMCID: PMC9655335
- DOI: 10.3390/ijms232113389
METCAM/MUC18 Plays a Tumor Suppressor Role in the Development of Nasopharyngeal Carcinoma Type I
Abstract
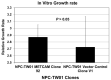
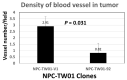
From previous studies of negatively correlating the expression of human METCAM/MUC18 with the pathology of nasopharyngeal carcinoma (NPC), we have suggested that human METCAM/MUC18 (huMETCAM/MUC18) might play a tumor suppressor role in the development of nasopharyngeal carcinoma. To scrutinize this hypothesis, we investigated the effects of huMETCAM/MUC18's over-expression on in vitro cellular behavior and on the in vivo tumorigenesis of one NPC cell line (NPC-TW01). HuMETCAM/MUC18 cDNA was first transfected into the NPC-TW01 cell line, which was established from NPC type I, and many G418-resistant clones were obtained. Then, two NPC-TW01 clones, which expressed high and medium levels of huMETCAM/MUC18, respectively, and one empty vector (control) clone were used to test the effects of huMETCAM/MUC18's over-expression on in vitro behaviors and on in vivo tumorigenesis (via subcutaneous injection) in athymic nude mice (Balb/cAnN.Cg-Foxnlnu/Cr1Nar1). The time course of tumor proliferation and the final tumor weights were determined. Tumor sections were used for the histology and immunohistochemistry (IHC) studies. Tumor lysates were used for determining the expression levels of huMETCAM/MUC18 and various downstream key effectors. HuMETCAM/MUC18's over-expression reduced in vitro motility and invasiveness and altered growth behaviors in 3D basement membrane culture assays, and it decreased the in vivo tumorigenicity of the NPC-TW01 cells. The tumor cells from a high-expressing clone were clustered and confined in small areas, whereas those from a vector control clone were more spread out, suggesting that the tumor cells from the high-expressing clone appeared to stay dormant in micro-clusters. Expression levels of the proliferation index, an index of the metabolic switch to aerobic glycolysis, angiogenesis indexes, and survival pathway indexes were reduced, whereas the pro-apoptosis index increased in the corresponding tumors. The over-expression of huMETCAM/MUC18 in the NPC-TW01 cells decreased the epithelial-to-mesenchymal transition and the in vitro and in vitro tumorigenesis, suggesting that it plays a tumor suppressor role in the development of type I NPC, perhaps by increasing apoptosis and decreasing angiogenesis, proliferation, and the metabolic switch to aerobic glycolysis.
Keywords: 3D basement membrane culture assay; HuMETCAM/MUC18; NPC-TW01; athymic nude mouse model; histology and immunohistochemistry; migration and invasiveness; nasopharyngeal carcinoma type I; tumor development; tumor suppression mechanism.
Conflict of interest statement
All authors declare that they have no conflict of interest.
Figures







Similar articles
-
METCAM/MUC18 augments migration, invasion, and tumorigenicity of human breast cancer SK-BR-3 cells.Gene. 2012 Jan 15;492(1):229-38. doi: 10.1016/j.gene.2011.10.024. Epub 2011 Oct 26. Gene. 2012. PMID: 22057013
-
Significance of expression of human METCAM/MUC18 in nasopharyngeal carcinomas and metastatic lesions.Asian Pac J Cancer Prev. 2014;15(1):245-52. doi: 10.7314/apjcp.2014.15.1.245. Asian Pac J Cancer Prev. 2014. PMID: 24528033
-
METCAM/MUC18 is a novel tumor and metastasis suppressor for the human ovarian cancer SKOV3 cells.BMC Cancer. 2016 Feb 22;16:136. doi: 10.1186/s12885-016-2181-9. BMC Cancer. 2016. PMID: 26906545 Free PMC article.
-
METCAM/MUC18 promoted tumorigenesis of human breast cancer SK-BR-3 cells in a dosage-specific manner.Taiwan J Obstet Gynecol. 2016 Apr;55(2):202-12. doi: 10.1016/j.tjog.2016.02.010. Taiwan J Obstet Gynecol. 2016. PMID: 27125403
-
METCAM/MUC18 Decreases the Malignant Propensity of Human Ovarian Carcinoma Cells.Int J Mol Sci. 2018 Sep 29;19(10):2976. doi: 10.3390/ijms19102976. Int J Mol Sci. 2018. PMID: 30274262 Free PMC article. Review.
Cited by
-
Angiogenesis in nasopharyngeal carcinoma: insights, imaging, and therapeutic strategies.Front Oncol. 2024 May 28;14:1331064. doi: 10.3389/fonc.2024.1331064. eCollection 2024. Front Oncol. 2024. PMID: 38863627 Free PMC article. Review.
-
METCAM Is a Potential Biomarker for Predicting the Malignant Propensity of and as a Therapeutic Target for Prostate Cancer.Biomedicines. 2023 Jan 13;11(1):205. doi: 10.3390/biomedicines11010205. Biomedicines. 2023. PMID: 36672713 Free PMC article. Review.
References
-
- Ho J.H.C. Current knowledge of the epidemiology of nasopharyngeal carcinoma (NPC) In: Biggs P., de The’ G., Payne L., editors. Oncogenesis and Herpesviruses. IARC; Lyon, France: 1972. pp. 357–366.
-
- Yu Y., Dong W., Zhou X., Li S. The significance of soluble intercellular adhesion molecule 1 (ICAM-1) and transformation growth factor alpha in patients with nasopharyngeal carcinomas. Acta Otolaryngol. Head Neck Surg. 2004;130:1205–1208. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

