Potential of Vitamin B6 Dioxime Analogues to Act as Cholinesterase Ligands
- PMID: 36362178
- PMCID: PMC9655973
- DOI: 10.3390/ijms232113388
Potential of Vitamin B6 Dioxime Analogues to Act as Cholinesterase Ligands
Abstract
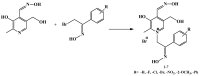
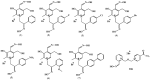
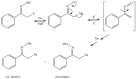
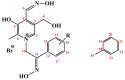
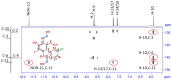
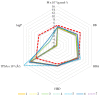
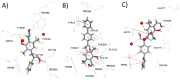
Seven pyridoxal dioxime quaternary salts (1-7) were synthesized with the aim of studying their interactions with human acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). The synthesis was achieved by the quaternization of pyridoxal monooxime with substituted 2-bromoacetophenone oximes (phenacyl bromide oximes). All compounds, prepared in good yields (43-76%) and characterized by 1D and 2D NMR spectroscopy, were evaluated as reversible inhibitors of cholinesterase and/or reactivators of enzymes inhibited by toxic organophosphorus compounds. Their potency was compared with that of their monooxime analogues and medically approved oxime HI-6. The obtained pyridoxal dioximes were relatively weak inhibitors for both enzymes (Ki = 100-400 µM). The second oxime group in the structure did not improve the binding compared to the monooxime analogues. The same was observed for reactivation of VX-, tabun-, and paraoxon-inhibited AChE and BChE, where no significant efficiency burst was noted. In silico analysis and molecular docking studies connected the kinetic data to the structural features of the tested compound, showing that the low binding affinity and reactivation efficacy may be a consequence of a bulk structure hindering important reactive groups. The tested dioximes were non-toxic to human neuroblastoma cells (SH-SY5Y) and human embryonal kidney cells (HEK293).
Keywords: AChE; BChE; cytotoxicity; dioxime; molecular docking; pyridoxal oxime; reactivation; reversible inhibition.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
New Heterostilbene and Triazole Oximes as Potential CNS-Active and Cholinesterase-Targeted Therapeutics.Biomolecules. 2024 Jun 11;14(6):679. doi: 10.3390/biom14060679. Biomolecules. 2024. PMID: 38927082 Free PMC article.
-
Pyridoxal oxime derivative potency to reactivate cholinesterases inhibited by organophosphorus compounds.Toxicol Lett. 2016 Nov 16;262:114-122. doi: 10.1016/j.toxlet.2016.09.015. Epub 2016 Sep 29. Toxicol Lett. 2016. PMID: 27693733
-
Synthesis and evaluation of novel analogues of vitamin B6 as reactivators of tabun and paraoxon inhibited acetylcholinesterase.Chem Biol Interact. 2010 Sep 6;187(1-3):234-7. doi: 10.1016/j.cbi.2010.02.004. Epub 2010 Feb 6. Chem Biol Interact. 2010. PMID: 20144593
-
Reactivators of butyrylcholinesterase inhibited by organophosphorus compounds.Bioorg Chem. 2024 Sep;150:107526. doi: 10.1016/j.bioorg.2024.107526. Epub 2024 Jun 4. Bioorg Chem. 2024. PMID: 38878749 Review.
-
The development of new oximes and the evaluation of their reactivating, therapeutic and neuroprotective efficacy against tabun.Mini Rev Med Chem. 2008 Oct;8(11):1134-43. doi: 10.2174/138955708785909871. Mini Rev Med Chem. 2008. PMID: 18855728 Review.
Cited by
-
New Heterostilbene and Triazole Oximes as Potential CNS-Active and Cholinesterase-Targeted Therapeutics.Biomolecules. 2024 Jun 11;14(6):679. doi: 10.3390/biom14060679. Biomolecules. 2024. PMID: 38927082 Free PMC article.
-
Application of α-bromination reaction on acetophenone derivatives in experimental teaching: a chemical innovation experiment engaging junior undergraduates.BMC Chem. 2024 Feb 21;18(1):38. doi: 10.1186/s13065-024-01145-y. BMC Chem. 2024. PMID: 38383516 Free PMC article.
References
-
- Timperley C., Forman J., Abdollahi M., Al-Amri A., Alonso I., Baulig A., Borrett V., Cariño F., Curty C., Gonzalez D., et al. Advice from the Scientific Advisory Board of the Organisation for the Prohibition of Chemical Weapons on isotopically labelled chemicals and stereoisomers in relation to the Chemical Weapons Convention. Pure Appl. Chem. 2018;90:1647–1670. doi: 10.1515/pac-2018-0803. - DOI
-
- Li B., Stribley J.A., Ticu A., Xie W., Schopfer L.M., Hammond P., Brimijoin S., Hinrichs S.H., Lockridge O. Abundant tissue butyrylcholinesterase and its possible function in the acetylcholinesterase knockout mouse. J. Neurochem. 2000;75:1320–1331. doi: 10.1046/j.1471-4159.2000.751320.x. - DOI - PubMed
-
- Zorbaz T., Malinak D., Hofmanova T., Maraković N., Žunec S., Maček Hrvat N., Andrys R., Psotka M., Zandona A., Svobodova J., et al. Halogen substituents enhance oxime nucleophilicity for reactivation of cholinesterases inhibited by nerve agents. Eur. J. Med. Chem. 2022;238:114377. doi: 10.1016/j.ejmech.2022.114377. - DOI - PubMed
-
- Semenov V.E., Zueva I.V., Mukhamedyarov M.A., Lushchekina S.V., Petukhova E.O., Gubaidullina L.M., Krylova E.S., Saifina L.F., Lenina O.A., Petrov K.A. Novel acetylcholinesterase inhibitors based on uracil moiety for possible treatment of Alzheimer disease. Molecules. 2020;25:4191. doi: 10.3390/molecules25184191. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

