Characterization of BCMA Expression in Circulating Rare Single Cells of Patients with Plasma Cell Neoplasms
- PMID: 36362214
- PMCID: PMC9658574
- DOI: 10.3390/ijms232113427
Characterization of BCMA Expression in Circulating Rare Single Cells of Patients with Plasma Cell Neoplasms
Abstract
B-cell maturation antigen (BCMA), a key regulator of B-cell proliferation and survival, is highly expressed in almost all cases of plasma cell neoplasms and B-lymphoproliferative malignancies. BCMA is a robust biomarker of plasma cells and a therapeutic target with substantial clinical significance. However, the expression of BCMA in circulating tumor cells of patients with hematological malignancies has not been validated for the detection of circulating plasma and B cells. The application of BCMA as a biomarker in single-cell detection and profiling of circulating tumor cells in patients' blood could enable early disease profiling and therapy response monitoring. Here, we report the development and validation of a slide-based immunofluorescence assay (i.e., CD138, BCMA, CD45, DAPI) for enrichment-free detection, quantification, and morphogenomic characterization of BCMA-expressing cells in patients (N = 9) with plasma cell neoplasms. Varying morphological subtypes of circulating BCMA-expressing cells were detected across the CD138(+/-) and CD45(+/-) compartments, representing candidate clonotypic post-germinal center B cells, plasmablasts, and both normal and malignant plasma cells. Genomic analysis by single-cell sequencing and correlation to clinical FISH cytogenetics provides validation, with data showing that patients across the different neoplastic states carry both normal and altered BCMA-expressing cells. Furthermore, altered cells harbor cytogenetic events detected by clinical FISH. The reported enrichment-free liquid biopsy approach has potential applications as a single-cell methodology for the early detection of BCMA+ B-lymphoid malignancies and in monitoring therapy response for patients undergoing anti-BCMA treatments.
Keywords: BCMA; HDSCA; bone marrow aspirate; circulating plasma cells; liquid biopsy; morphogenomics; multimodal data; multiple myeloma; peripheral blood; rare single cell.
Conflict of interest statement
The authors declare the following conflicts of interest: P.K. holds ownership interests in Epic Sciences and is a paid consultant/advisory board member for Epic Sciences. C.R.V., N.M, R.N., J.H., and P.K. are royalty recipients for related technologies licensed to Epic Sciences for development.
Figures
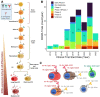
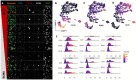
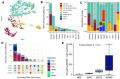

References
-
- Madry C., Laabi Y., Callebaut I., Roussel J., Hatzoglou A., Le Coniat M., Mornon J.P., Berger R., Tsapis A. The characterization of murine BCMA gene defines it as a new member of the tumor necrosis factor receptor superfamily. Int. Immunol. 1998;10:1693–1702. doi: 10.1093/intimm/10.11.1693. - DOI - PubMed
-
- Hatzoglou A., Roussel J., Bourgeade M.-F., Rogier E., Madry C., Inoue J., Devergne O., Tsapis A. TNF Receptor Family Member BCMA (B Cell Maturation) Associates with TNF Receptor-Associated Factor (TRAF) 1, TRAF2, and TRAF3 and Activates NF-κB, Elk-1, c-Jun N-Terminal Kinase, and p38 Mitogen-Activated Protein Kinase. J. Immunol. 2000;165:1322–1330. doi: 10.4049/jimmunol.165.3.1322. - DOI - PubMed
MeSH terms
Substances
Grants and funding
- P30 CA016672/CA/NCI NIH HHS/United States
- Eric Yang/Dornsife Summer Undergraduate Research Fun
- USC CSI-Cancer (institutional support)/USC Michelson Center Convergent Science Institute in Cancer
- USC CSI-Cancer/Winnie and James Hart Endowed Fellowship
- 5P30CA014089-40/USC Norris Comprehensive Cancer Center (CORE) Support
- Elisabet E Manasanch/University of Texas MD Anderson Moon Shot Program
- USC CSI-Cancer/Schlegel Family Fellowship Fu
- P30 CA014089/CA/NCI NIH HHS/United States
- KL2TR001854/TR/NCATS NIH HHS/United States
- Elisabet E Manasanch/Paula and Rodger Riney Foundation
- Specialized Center of Research/Leukemia and Lymphoma Society
- APMMR/Dr. Miriam and Sheldon G. Adelson Medical Research Foundation
- KL2 TR001854/TR/NCATS NIH HHS/United States
- Eric Yang/USC Provost Research Fellow
- Peter Kuhn/Vassiliadis Research Fund
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

