The IL-33/ST2 Pathway in Cerebral Malaria
- PMID: 36362246
- PMCID: PMC9658244
- DOI: 10.3390/ijms232113457
The IL-33/ST2 Pathway in Cerebral Malaria
Abstract
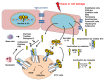
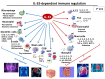
Interleukin-33 (IL-33) is an immunomodulatory cytokine which plays critical roles in tissue function and immune-mediated diseases. IL-33 is abundant within the brain and spinal cord tissues where it acts as a key cytokine to coordinate the exchange between the immune and central nervous system (CNS). In this review, we report the recent advances to our knowledge regarding the role of IL-33 and of its receptor ST2 in cerebral malaria, and in particular, we highlight the pivotal role that IL-33/ST2 signaling pathway could play in brain and cerebrospinal barriers permeability. IL-33 serum levels are significantly higher in children with severe Plasmodium falciparum malaria than children without complications or noninfected children. IL-33 levels are correlated with parasite load and strongly decrease with parasite clearance. We postulate that sequestration of infected erythrocytes or merozoites liberation from schizonts could amplify IL-33 production in endothelial cells, contributing either to malaria pathogenesis or recovery.
Keywords: IL-33; Plasmodium; ST2; astrocytes; blood–brain barrier; central nervous system; cerebral malaria; endothelial; inflammation; red blood cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bagot S., Nogueira F., Collette A., do Rosario V., Lemonier F., Cazenave P.-A., Pied S. Comparative Study of Brain CD8+ T Cells Induced by Sporozoites and Those Induced by Blood-Stage Plasmodium Berghei ANKA Involved in the Development of Cerebral Malaria. Infect. Immun. 2004;72:2817–2826. doi: 10.1128/IAI.72.5.2817-2826.2004. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

