Neurodegenerative Disorder Risk in Krabbe Disease Carriers
- PMID: 36362324
- PMCID: PMC9654610
- DOI: 10.3390/ijms232113537
Neurodegenerative Disorder Risk in Krabbe Disease Carriers
Abstract
Krabbe disease (KD) is a rare autosomal recessive disorder caused by mutations in the galactocerebrosidase gene (GALC). Defective GALC causes aberrant metabolism of galactolipids present almost exclusively in myelin, with consequent demyelinization and neurodegeneration of the central and peripheral nervous system (NS). KD shares some similar features with other neuropathies and heterozygous carriers of GALC mutations are emerging with an increased risk in developing NS disorders. In this work, we set out to identify possible variations in the proteomic profile of KD-carrier brain to identify altered pathways that may imbalance its homeostasis and that may be associated with neurological disorders. The differential analysis performed on whole brains from 33-day-old twitcher (galc -/-), heterozygous (galc +/-), and wild-type mice highlighted the dysregulation of several multifunctional factors in both heterozygous and twitcher mice. Notably, the KD-carrier mouse, despite its normal phenotype, presents the deregulation of vimentin, receptor of activated protein C kinase 1 (RACK1), myelin basic protein (MBP), 2',3'-cyclic-nucleotide 3'-phosphodiesterase (CNP), transitional endoplasmic reticulum ATPase (VCP), and N-myc downstream regulated gene 1 protein (NDRG1) as well as changes in the ubiquitinated-protein pattern. Our findings suggest the carrier may be affected by dysfunctions classically associated with neurodegeneration: (i) alteration of (mechano) signaling and intracellular trafficking, (ii) a generalized affection of proteostasis and lipid metabolism, with possible defects in myelin composition and turnover, and (iii) mitochondrion and energy supply dysfunctions.
Keywords: Alzheimer disease; Lewy body; Parkinson disease; demyelination; globoid cell leukodystrophy; multiple sclerosis; neurodegeneration; neuroinflammation; ubiquitin dependent degradation.
Conflict of interest statement
The authors declare that they have no current or potential conflict of interest regarding this article.
Figures
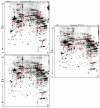
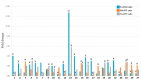
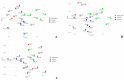


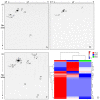
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

