Application of Computational Biology and Artificial Intelligence in Drug Design
- PMID: 36362355
- PMCID: PMC9658956
- DOI: 10.3390/ijms232113568
Application of Computational Biology and Artificial Intelligence in Drug Design
Abstract
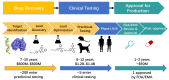
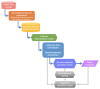
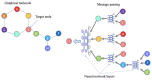
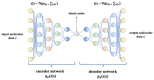
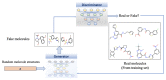
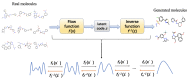
Traditional drug design requires a great amount of research time and developmental expense. Booming computational approaches, including computational biology, computer-aided drug design, and artificial intelligence, have the potential to expedite the efficiency of drug discovery by minimizing the time and financial cost. In recent years, computational approaches are being widely used to improve the efficacy and effectiveness of drug discovery and pipeline, leading to the approval of plenty of new drugs for marketing. The present review emphasizes on the applications of these indispensable computational approaches in aiding target identification, lead discovery, and lead optimization. Some challenges of using these approaches for drug design are also discussed. Moreover, we propose a methodology for integrating various computational techniques into new drug discovery and design.
Keywords: artificial intelligence-aided drug design (AIDD); computational biology; computer-aided drug design (CADD); deep learning.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Kalyan K. Pharmaceutical Medicine and Translational Clinical Research. Curr. Sci. 2018;115:1403.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

