L-Type Ca2+ Channel Inhibition Rescues the LPS-Induced Neuroinflammatory Response and Impairments in Spatial Memory and Dendritic Spine Formation
- PMID: 36362394
- PMCID: PMC9655622
- DOI: 10.3390/ijms232113606
L-Type Ca2+ Channel Inhibition Rescues the LPS-Induced Neuroinflammatory Response and Impairments in Spatial Memory and Dendritic Spine Formation
Abstract
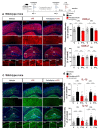
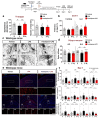
Ca2+ signaling is implicated in the transition between microglial surveillance and activation. Several L-type Ca2+ channel blockers (CCBs) have been shown to ameliorate neuroinflammation by modulating microglial activity. In this study, we examined the effects of the L-type CCB felodipine on LPS-mediated proinflammatory responses. We found that felodipine treatment significantly diminished LPS-evoked proinflammatory cytokine levels in BV2 microglial cells in an L-type Ca2+ channel-dependent manner. In addition, felodipine leads to the inhibition of TLR4/AKT/STAT3 signaling in BV2 microglial cells. We further examined the effects of felodipine on LPS-stimulated neuroinflammation in vivo and found that daily administration (3 or 7 days, i.p.) significantly reduced LPS-mediated gliosis and COX-2 and IL-1β levels in C57BL/6 (wild-type) mice. Moreover, felodipine administration significantly reduced chronic neuroinflammation-induced spatial memory impairment, dendritic spine number, and microgliosis in C57BL/6 mice. Taken together, our results suggest that the L-type CCB felodipine could be repurposed for the treatment of neuroinflammation/cognitive function-associated diseases.
Keywords: Ca2+ channel blocker; LPS; felodipine; gliosis; neuroinflammation; spatial memory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







References
MeSH terms
Substances
Grants and funding
- 22-BR-02-03, 22-BR-03-05, 22-BR-04-01, 22-BR-02-12, H.S.H./KBRI basic research program through KBRI funded by the Ministry of Science, ICT & Future Planning
- 2021R1F1A1057865, J.K./National Research Foundation of Korea
- 20190058/Whanin Pharm Co., Ltd.
- CCL22061-100, H.S.H/National Research Council of Science & Technology (NST) grant funded by the Korean govern-ment
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

