Magnetic Multi-Enzymatic System for Cladribine Manufacturing
- PMID: 36362425
- PMCID: PMC9658597
- DOI: 10.3390/ijms232113634
Magnetic Multi-Enzymatic System for Cladribine Manufacturing
Abstract
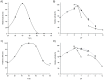
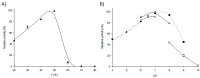
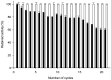
Enzyme-mediated processes have proven to be a valuable and sustainable alternative to traditional chemical methods. In this regard, the use of multi-enzymatic systems enables the realization of complex synthetic schemes, while also introducing a number of additional advantages, including the conversion of reversible reactions into irreversible processes, the partial or complete elimination of product inhibition problems, and the minimization of undesirable by-products. In addition, the immobilization of biocatalysts on magnetic supports allows for easy reusability and streamlines the downstream process. Herein we have developed a cascade system for cladribine synthesis based on the sequential action of two magnetic biocatalysts. For that purpose, purine 2'-deoxyribosyltransferase from Leishmania mexicana (LmPDT) and Escherichia coli hypoxanthine phosphoribosyltransferase (EcHPRT) were immobilized onto Ni2+-prechelated magnetic microspheres (MagReSyn®NTA). Among the resulting derivatives, MLmPDT3 (activity: 11,935 IU/gsupport, 63% retained activity, operational conditions: 40 °C and pH 5-7) and MEcHPRT3 (12,840 IU/gsupport, 45% retained activity, operational conditions: pH 5-8 and 40-60 °C) emerge as optimal catalysts for further synthetic application. Moreover, the MLmPDT3/MEcHPRT3 system was biochemically characterized and successfully applied to the one-pot synthesis of cladribine under various conditions. This methodology not only displayed a 1.67-fold improvement in cladribine synthesis (compared to MLmPDT3), but it also implied a practically complete transformation of the undesired by-product into a high-added-value product (90% conversion of Hyp into IMP). Finally, MLmPDT3/MEcHPRT3 was reused for 16 cycles, which displayed a 75% retained activity.
Keywords: cascade synthesis; enzyme immobilization; magnetic catalysts; nucleoside analogues; transglycosylation reaction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Sustainable production of nucleoside analogues by a high-efficient purine 2'-deoxyribosyltransferase immobilized onto Ni2+ chelate magnetic microparticles.Bioresour Technol. 2019 Oct;289:121772. doi: 10.1016/j.biortech.2019.121772. Epub 2019 Jul 7. Bioresour Technol. 2019. PMID: 31307865
-
Sustainable synthesis of uridine-5'-monophosphate analogues by immobilized uracil phosphoribosyltransferase from Thermus thermophilus.Biochim Biophys Acta Proteins Proteom. 2020 Jan;1868(1):140251. doi: 10.1016/j.bbapap.2019.07.004. Epub 2019 Jul 9. Biochim Biophys Acta Proteins Proteom. 2020. PMID: 31299354
-
2'-Deoxyribosyltransferase from Leishmania mexicana, an efficient biocatalyst for one-pot, one-step synthesis of nucleosides from poorly soluble purine bases.Appl Microbiol Biotechnol. 2017 Oct;101(19):7187-7200. doi: 10.1007/s00253-017-8450-y. Epub 2017 Aug 7. Appl Microbiol Biotechnol. 2017. PMID: 28785897
-
Magnetic micro-macro biocatalysts applied to industrial bioprocesses.Bioresour Technol. 2021 Feb;322:124547. doi: 10.1016/j.biortech.2020.124547. Epub 2020 Dec 16. Bioresour Technol. 2021. PMID: 33352394 Review.
-
Application of magnetic nanoparticles in smart enzyme immobilization.Biotechnol Lett. 2016 Feb;38(2):223-33. doi: 10.1007/s10529-015-1977-z. Epub 2015 Oct 15. Biotechnol Lett. 2016. PMID: 26472272 Review.
Cited by
-
Novel Directed Enzyme Prodrug Therapy for Cancer Treatment Based on 2'-Deoxyribosyltransferase-Conjugated Magnetic Nanoparticles.Biomolecules. 2024 Jul 24;14(8):894. doi: 10.3390/biom14080894. Biomolecules. 2024. PMID: 39199282 Free PMC article.
-
Enzymatic Protein Immobilization on Amino-Functionalized Nanoparticles.Molecules. 2023 Jan 2;28(1):379. doi: 10.3390/molecules28010379. Molecules. 2023. PMID: 36615576 Free PMC article.
-
Engineering a Bifunctional Fusion Purine/Pyrimidine Nucleoside Phosphorylase for the Production of Nucleoside Analogs.Biomolecules. 2024 Sep 23;14(9):1196. doi: 10.3390/biom14091196. Biomolecules. 2024. PMID: 39334962 Free PMC article.
-
Biocatalysis: An Eco-Friendly Scenario for the Manufacturing of APIs.Int J Mol Sci. 2023 Feb 24;24(5):4474. doi: 10.3390/ijms24054474. Int J Mol Sci. 2023. PMID: 36901905 Free PMC article.
References
-
- Fernández-Lucas J., Camarasa M.J., editors. Enzymatic and Chemical Synthesis of Nucleic acid Derivatives. John Wiley & Sons; Hoboken, NJ, USA: 2019.
-
- Lapponi M.J., Rivero C.W., Zinni M.A., Britos C.N., Trelles J.A. New developments in nucleoside analogues biosynthesis: A review. J. Mol. Catal. B Enzym. 2016;133:218–233. doi: 10.1016/j.molcatb.2016.08.015. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

