Proactive versus Rank-Down Topical Corticosteroid Therapy for Maintenance of Remission in Pediatric Atopic Dermatitis: A Randomized, Open-Label, Active-Controlled, Parallel-Group Study (Anticipate Study)
- PMID: 36362704
- PMCID: PMC9658234
- DOI: 10.3390/jcm11216477
Proactive versus Rank-Down Topical Corticosteroid Therapy for Maintenance of Remission in Pediatric Atopic Dermatitis: A Randomized, Open-Label, Active-Controlled, Parallel-Group Study (Anticipate Study)
Abstract
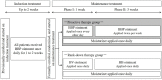
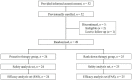
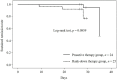
Topical corticosteroids are used as first-line treatment for atopic dermatitis (AD). Regarding the maintenance of remission achieved by topical corticosteroids, no previous studies have compared proactive therapy with rank-down therapy. We compared their efficacy and safety in Japanese children with moderate to severe AD. Patients who had achieved remission with a very strong topical corticosteroid were randomized to 4-week maintenance treatment with either intermittent use of the same drug (proactive therapy) or daily use of a strong topical corticosteroid for 1 week followed by daily use of a medium-potency topical corticosteroid for 3 weeks (rank-down therapy); 49 patients were randomized (proactive therapy, n = 24; rank-down therapy, n = 25). During maintenance treatment, the relapse rate was 8.33% in the proactive therapy group and 20.0% in the rank-down therapy group (p = 0.0859). The mean (±standard deviation) itching score on a numerical rating scale in the rank-down therapy group increased significantly from 2.5 ± 1.9 to 3.6 ± 2.6 (p = 0.0438). Adverse events occurred in 2 patients receiving proactive therapy and 3 patients receiving rank-down therapy. Proactive therapy appears to be as safe as rank-down therapy and may be more effective for itch in pediatric AD in remission.
Keywords: atopic dermatitis; pediatric; proactive therapy; rank-down therapy; topical corticosteroids.
Conflict of interest statement
K.K. and S.Y. have no conflicts of interest to declare. H.S. has received payment or honoraria for lectures/speaking from Mitsubishi Tanabe Pharma Corporation, Taiho Pharmaceutical Co., Ltd., AbbVie GK, Sanofi K.K., Torii Pharmaceutical Co., Ltd., Maruho Co., Ltd., Kyowa Kirin Co., Ltd., Japan Tobacco Inc., Eli Lilly Japan K.K., LEO Pharma K.K., Otsuka Pharmaceutical Co., Ltd., and Novartis Pharma K.K.; has received funds for commissioned or joint research from AbbVie GK, Maruho Co., Ltd., and LEO Pharma K.K. and has received scholarship grants from Taiho Pharmaceutical Co., Ltd., Maruho Co., Ltd., Eisai Co., Ltd., and TOKIWA Pharmaceutical Co., Ltd. Y.T. has received fees for meeting attendance or lectures from Mitsubishi Tanabe Pharma Corporation, Sanofi K.K., Maruho Co., Ltd., Kyowa Kirin Co., Ltd., and Igaku-Shoin Ltd. and has received funds for commissioned or joint research from Novartis Pharma K.K. and LEO Pharma K.K. J.S. has received payment or honoraria for lectures/speaking from Maruho Co., Ltd. M.O. has received research grants and/or personal fees from AbbVie GK, Bristol Myers Squibb, Eisai Co., Ltd., Eli Lilly Japan K.K., Janssen Pharmaceuticals, LEO Pharma K.K., Maruho Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Novartis Pharma K.K., Pfizer, Sanofi, Taiho Pharmaceutical Co., Ltd., and Torii Pharmaceutical Co., Ltd.
Figures
References
-
- Jackson K.D., Howie L.D., Akinbami L.J. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief. 2013;121:1–8. - PubMed
-
- Eichenfield L.F., Tom W.L., Berger T.G., Krol A., Paller A.S., Schwarzenberger K., Bergman J.N., Chamlin S.L., Cohen D.E., Cooper K.D., et al. Guidelines of care for the management of atopic dermatitis: Section 2. Management and treatment of atopic dermatitis with topical therapies. J. Am. Acad. Dermatol. 2014;71:116–132. doi: 10.1016/j.jaad.2014.03.023. - DOI - PMC - PubMed
-
- Wollenberg A., Barbarot S., Bieber T., Christen-Zaech S., Deleuran M., Fink-Wagner A., Gieler U., Girolomoni G., Lau S., Muraro A., et al. Consensus-based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children: Part I. J. Eur. Acad. Dermatol. Venereol. 2018;32:657–682. doi: 10.1111/jdv.14891. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

