Three-Country Snapshot of Ornithine Transcarbamylase Deficiency
- PMID: 36362876
- PMCID: PMC9695856
- DOI: 10.3390/life12111721
Three-Country Snapshot of Ornithine Transcarbamylase Deficiency
Abstract
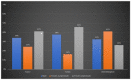
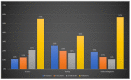
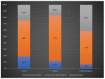
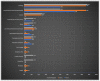
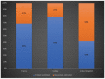
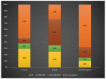
X-linked ornithine transcarbamylase deficiency (OTCD) is the most common urea cycle defect. The disease severity ranges from asymptomatic carrier state to severe neonatal presentation with hyperammonaemic encephalopathy. We audited the diagnosis and management of OTCD, using an online 12-question-survey that was sent to 75 metabolic centres in Turkey, France and the UK. Thirty-nine centres responded and 495 patients were reported in total. A total of 208 French patients were reported, including 71 (34%) males, 86 (41%) symptomatic and 51 (25%) asymptomatic females. Eighty-five Turkish patients included 32 (38%) males, 39 (46%) symptomatic and 14 (16%) asymptomatic females. Out of the 202 UK patients, 66 (33%) were male, 83 (41%) asymptomatic and 53 (26%) symptomatic females. A total of 19%, 12% and 7% of the patients presented with a neonatal-onset phenotype in France, Turkey and the UK, respectively. Vomiting, altered mental status and encephalopathy were the most common initial symptoms in all three countries. While 69% in France and 79% in Turkey were receiving protein restriction, 42% were on a protein-restricted diet in the UK. A total of 76%, 47% and 33% of patients were treated with ammonia scavengers in Turkey, France and the UK, respectively. The findings of our audit emphasize the differences and similarities in manifestations and management practices in three countries.
Keywords: ammonia scavengers; asymptomatic; hyperammonaemia; late-onset; liver transplantation; neonatal-onset; ornithine transcarbamylase deficiency; protein restriction.
Conflict of interest statement
PG is an academic co-founder of Bloomsbury Genetic Therapies, UCL spinout developing a gene programme in OTC deficiency.
Figures
References
-
- Seminara J., Tuchman M., Krivitzky L., Krischer J., Lee H.-S., LeMons C., Baumgartner M., Cederbaum S., Diaz G.A., Feigenbaum A., et al. Establishing a consortium for the study of rare diseases: The Urea Cycle Disorders Consortium. Mol. Genet. Metab. 2010;100((Suppl. 1)):S97–S105. doi: 10.1016/j.ymgme.2010.01.014. - DOI - PMC - PubMed
-
- Brusilow S.W., Maestri N.E. Urea cycle disorders: Diagnosis, pathophysiology, and therapy. Adv. Pediatr. 1996;43:127–170. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

