Characterization of Commercial Polymer-Carbon Composite Bipolar Plates Used in PEM Fuel Cells
- PMID: 36363605
- PMCID: PMC9695731
- DOI: 10.3390/membranes12111050
Characterization of Commercial Polymer-Carbon Composite Bipolar Plates Used in PEM Fuel Cells
Abstract
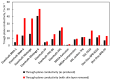
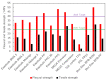
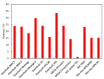
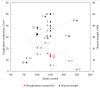
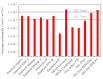
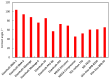
Bipolar plates represent a crucial component of the PEM fuel cell stack. Polymer-carbon composites are recognized as state-of-the-art materials for bipolar plate manufacturing, but their use involves a compromise between electrical and heat conductivity, mechanical strength and costs. Thus, all key parameters must be considered when selecting a suitable plate satisfying the demands of the desired application. However, data relevant to commercial materials for such selection are scarce in the open literature. To address this issue, 13 commercially available polymer-carbon composites are characterised in terms of the following parameters: through-plane conductivity, hydrogen permeability, mechanical strength, water uptake, density, water contact angle and chemical stability. None of the materials tested reached the DOE target for electrical conductivity, while five of the materials met the target for flexural strength. The overall best-performing material showed a conductivity value of 50.4 S·cm-1 and flexural strength of 40.1 MPa. The data collected provide important supporting information in selecting the materials most suitable for the desired application. In addition, the key parameters determined for each bipolar plate supply important input parameters for the mathematical modelling of fuel cells.
Keywords: PEM; bipolar plate; carbon–polymer composites; electrical conductivity; fuel cell; material selection; mechanical strength.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Ajanovic A., Haas R. Prospects and impediments for hydrogen and fuel cell vehicles in the transport sector. Int. J. Hydrogen Energy. 2021;46:10049–10058. doi: 10.1016/j.ijhydene.2020.03.122. - DOI
-
- Sun C., Negro E., Vezzù K., Pagot G., Cavinato G., Nale A., Herve Bang Y., Di Noto V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta. 2019;309:311–325. doi: 10.1016/j.electacta.2019.03.056. - DOI
-
- Nazir H., Muthuswamy N., Louis C., Jose S., Prakash J., Buan M.E.M., Flox C., Chavan S., Shi X., Kauranen P., et al. Is the H2 economy realizable in the foreseeable future? Part III: H2 usage technologies, applications, and challenges and opportunities. Int. J. Hydrogen Energy. 2020;45:28217–28239. doi: 10.1016/j.ijhydene.2020.07.256. - DOI - PMC - PubMed
-
- Guerrero Moreno N., Cisneros Molina M., Gervasio D., Pérez Robles J.F. Approaches to polymer electrolyte membrane fuel cells (PEMFCs) and their cost. Renew. Sustain. Energy Rev. 2015;52:897–906. doi: 10.1016/j.rser.2015.07.157. - DOI
-
- Thompson S.T., James B.D., Huya-Kouadio J.M., Houchins C., DeSantis D.A., Ahluwalia R., Wilson A.R., Kleen G., Papageorgopoulos D. Direct hydrogen fuel cell electric vehicle cost analysis: System and high-volume manufacturing description, validation, and outlook. J. Power Sources. 2018;399:304–313. doi: 10.1016/j.jpowsour.2018.07.100. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

