Computational Approaches to Alkaline Anion-Exchange Membranes for Fuel Cell Applications
- PMID: 36363606
- PMCID: PMC9693448
- DOI: 10.3390/membranes12111051
Computational Approaches to Alkaline Anion-Exchange Membranes for Fuel Cell Applications
Abstract
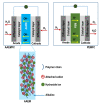
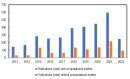
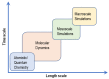
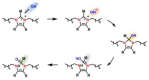
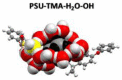
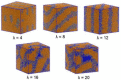
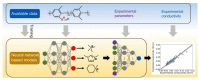
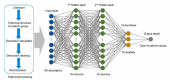
Anion-exchange membranes (AEMs) are key components in relatively novel technologies such as alkaline exchange-based membrane fuel cells and AEM-based water electrolyzers. The application of AEMs in these processes is made possible in an alkaline environment, where hydroxide ions (OH-) play the role of charge carriers in the presence of an electrocatalyst and an AEM acts as an electrical insulator blocking the transport of electrons, thereby preventing circuit break. Thus, a good AEM would allow the selective transport of OH- while preventing fuel (e.g., hydrogen, alcohol) crossover. These issues are the subjects of in-depth studies of AEMs-both experimental and theoretical studies-with particular emphasis on the ionic conductivity, ion exchange capacity, fuel crossover, durability, stability, and cell performance properties of AEMs. In this review article, the computational approaches used to investigate the properties of AEMs are discussed. The different modeling length scales are microscopic, mesoscopic, and macroscopic. The microscopic scale entails the ab initio and quantum mechanical modeling of alkaline AEMs. The mesoscopic scale entails using molecular dynamics simulations and other techniques to assess the alkaline electrolyte diffusion in AEMs, OH- transport and chemical degradation in AEMs, ion exchange capacity of an AEM, as well as morphological microstructures. This review shows that computational approaches can be used to investigate different properties of AEMs and sheds light on how the different computational domains can be deployed to investigate AEM properties.
Keywords: alkaline anion-exchange membranes; computational approaches; macroscopic; mesoscopic; microscopic.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Hickner M.A., Herring A.M., Coughlin E.B. Anion exchange membranes: Current status and moving forward. J. Polym. Sci. Part B Polym. Phys. 2013;51:1727–1735. doi: 10.1002/polb.23395. - DOI
-
- Vincent I., Kruger A., Bessarabov D. Development of efficient membrane electrode assembly for low cost hydrogen production by anion exchange membrane electrolysis. Int. J. Hydrogen Energy. 2017;42:10752–10761. doi: 10.1016/j.ijhydene.2017.03.069. - DOI
-
- Gao X., He L., Yu H., Xie F., Yang Y., Shao Z. The non-precious metal ORR catalysts for the anion exchange membrane fuel cells application: A numerical simulation and experimental study. Int. J. Hydrogen Energy. 2020;45:23353–23367. doi: 10.1016/j.ijhydene.2020.06.066. - DOI
-
- Zhang F., Li T., Chen W., Yan X., Wu X., Jiang X., Zhang Y., Wang X., He G. High-Performance Anion Exchange Membranes with Para-Type Cations on Electron-Withdrawing C=O Links Free Backbone. Macromolecules. 2020;53:10988–10997. doi: 10.1021/acs.macromol.0c01710. - DOI
Publication types
LinkOut - more resources
Full Text Sources

