Different Impacts of Heat-Killed and Viable Lactiplantibacillus plantarum TWK10 on Exercise Performance, Fatigue, Body Composition, and Gut Microbiota in Humans
- PMID: 36363775
- PMCID: PMC9692508
- DOI: 10.3390/microorganisms10112181
Different Impacts of Heat-Killed and Viable Lactiplantibacillus plantarum TWK10 on Exercise Performance, Fatigue, Body Composition, and Gut Microbiota in Humans
Abstract
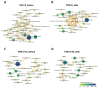
Lactiplantibacillus plantarum TWK10, a probiotic strain, has been demonstrated to improve exercise performance, regulate body composition, and ameliorate age-related declines. Here, we performed a comparative analysis of viable and heat-killed TWK10 in the regulation of exercise performance, body composition, and gut microbiota in humans. Healthy adults (n = 53) were randomly divided into three groups: Control, TWK10 (viable TWK10, 3 × 1011 colony forming units/day), and TWK10-hk (heat-killed TWK10, 3 × 1011 cells/day) groups. After six-week administration, both the TWK10 and TWK10-hk groups had significantly improved exercise performance and fatigue-associated features and reduced exercise-induced inflammation, compared with controls. Viable TWK10 significantly promoted improved body composition, by increasing muscle mass proportion and reducing fat mass. Gut microbiota analysis demonstrated significantly increasing trends in the relative abundances of Akkermansiaceae and Prevotellaceae in subjects receiving viable TWK10. Predictive metagenomic profiling revealed that heat-killed TWK10 administration significantly enhanced the signaling pathways involved in amino acid metabolisms, while glutathione metabolism, and ubiquinone and other terpenoid-quinone biosynthesis pathways were enriched by viable TWK10. In conclusion, viable and heat-killed TWK10 had similar effects in improving exercise performance and attenuating exercise-induced inflammatory responses as probiotics and postbiotics, respectively. Viable TWK10 was also highly effective in regulating body composition. The differences in efficacy between viable and heat-killed TWK10 may be due to differential impacts in shaping gut microbiota.
Keywords: Lactiplantibacillus plantarum TWK10; anti-fatigue; exercise performance; heat-killed; microbiota; postbiotics; probiotics; viable.
Conflict of interest statement
Author C.-C.L., Y.-C.L., M.-C.L., Y.-C.C., S.-Y.C., J.-S.L., and K.W. are employed by SYNBIO TECH INC. M.-C.L. and C.-C.H. declare that there are no conflict of interest.
Figures


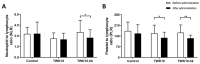



References
-
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. - DOI - PubMed
-
- Nagpal R., Kumar A., Kumar M., Behare P.V., Jain S., Yadav H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012;334:1–15. - PubMed
LinkOut - more resources
Full Text Sources
Medical

