Push-Pull Structures Based on 2-Aryl/thienyl Substituted Quinazolin-4(3 H)-ones and 4-Cyanoquinazolines
- PMID: 36363984
- PMCID: PMC9658551
- DOI: 10.3390/molecules27217156
Push-Pull Structures Based on 2-Aryl/thienyl Substituted Quinazolin-4(3 H)-ones and 4-Cyanoquinazolines
Abstract
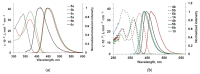
Design and synthesis of 2-(aryl/thiophen-2-yl)quinazolin-4(3H)-ones and 4-cyano-2-arylquinazolines with Et2N-, Ph2N- or carbazol-9-yl- electron donating fragment are described. The key photophysical properties of these compounds have been studied by UV/Vis absorption and fluorescence spectroscopy in solvents of different polarity (toluene and MeCN). 2-(Aryl/thiophen-2-yl)quinazolin-4(3H)-ones show fluorescence in blue-green region in toluene solution with quantum yields up to 89% in the case of 2-(4'-N,N-diphenylamino[1,1'-biphenyl]-4-yl)-quinazolin-4(3H)-one. Moreover, triphenylamino derivative based on quinazolin-4(3H)-one with para-phenylene linker displays the highest quantum yield of 40% in powder. The fluorescence QY of Et2N and Ph2N derivatives decrease when going from toluene to MeCN solution, whereas carbazol-9-yl counterparts demonstrate strengthening of intensity that emphasizes the strong influence of donor fragment nature on photophysical properties. 4-Cyanoquinazolines are less emissive in both solvents, as well as, in solid state. The introduction of cyano group into position 4 leads to orange/red colored powder and dual emission bands. Some molecules demonstrate the increase in emission intensity upon addition of water to MeCN solution. According to frontier molecular orbitals (HOMO, LUMO) calculations, the energy gap of 4-cyanoquinazoline decreases by more than 1 eV compared to quinazolin-4-one, that is consistent with experimental data.
Keywords: 2-(biphenyl)quinazoline; 2-thienylquinazoline; 4-cyanoquinazoline; donor–acceptor systems; fluorescence; quinazolin-4(3H)-one; π-linker.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Schramm S., Weiß D. Advances in Heterocyclic Chemistry. Volume 128. Elsevier Inc.; Amsterdam, The Netherlands: 2019. Fluorescent heterocycles: Recent trends and new developments; pp. 103–179.
-
- Soleymani M., Chegeni M. The Chemistry and Applications of the Quinoxaline Compounds. Curr. Org. Chem. 2019;23:1789–1827. doi: 10.2174/1385272823666190926094348. - DOI
-
- Lipunova G.N., Nosova E.V., Charushin V.N., Chupakhin O.N. Functionalized Quinazolines and Pyrimidines for Optoelectronic Materials. Curr. Org. Synth. 2018;15:793–814. doi: 10.2174/1570179415666180622123434. - DOI
-
- Nosova E.V., Achelle S., Lipunova G.N., Charushin V.N., Chupakhin O.N. Functionalized Benzazines as Luminescent Materials and Components for Optoelectronics. Russ. Chem. Rev. 2019;88:1128–1178. doi: 10.1070/RCR4887. - DOI
-
- Ermakova E.V., Cheprakov A.V., Bessmertnykh-Lemeune A. Aminoquinoxaline-Based Dual Colorimetric and Fluorescent Sensors for PH Measurement in Aqueous Media. Chemosensors. 2022;10:342. doi: 10.3390/chemosensors10080342. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

