Photocatalytic Water Splitting: How Far Away Are We from Being Able to Industrially Produce Solar Hydrogen?
- PMID: 36364002
- PMCID: PMC9657347
- DOI: 10.3390/molecules27217176
Photocatalytic Water Splitting: How Far Away Are We from Being Able to Industrially Produce Solar Hydrogen?
Abstract
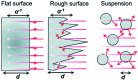
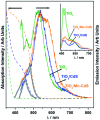
Solar water splitting (SWS) has been researched for about five decades, but despite successes there has not been a big breakthrough advancement. While the three fundamental steps, light absorption, charge carrier separation and diffusion, and charge utilization at redox sites are given a great deal of attention either separately or simultaneously, practical considerations that can help to increase efficiency are rarely discussed or put into practice. Nevertheless, it is possible to increase the generation of solar hydrogen by making a few little but important adjustments. In this review, we talk about various methods for photocatalytic water splitting that have been documented in the literature and importance of the thin film approach to move closer to the large-scale photocatalytic hydrogen production. For instance, when comparing the film form of the identical catalyst to the particulate form, it was found that the solar hydrogen production increased by up to two orders of magnitude. The major topic of this review with thin-film forms is, discussion on several methods of increased hydrogen generation under direct solar and one-sun circumstances. The advantages and disadvantages of thin film and particle technologies are extensively discussed. In the current assessment, potential approaches and scalable success factors are also covered. As demonstrated by a film-based approach, the local charge utilization at a zero applied potential is an appealing characteristic for SWS. Furthermore, we compare the PEC-WS and SWS for solar hydrogen generation and discuss how far we are from producing solar hydrogen on an industrial scale. We believe that the currently employed variety of attempts may be condensed to fewer strategies such as film-based evaluation, which will create a path to address the SWS issue and achieve sustainable solar hydrogen generation.
Keywords: hydrogen production; large scale evolution; photocatalytic water splitting; solar energy; thin films.
Conflict of interest statement
There are no conflict to declare.
Figures






References
-
- Escobedo Salas S., Serrano Rosales B., de Lasa H. Quantum Yield with Platinum Modified TiO2 Photocatalyst for Hydrogen Production. Appl. Catal. B Environ. 2013;140–141:523–536. doi: 10.1016/j.apcatb.2013.04.016. - DOI
-
- Abe R. Recent Progress on Photocatalytic and Photoelectrochemical Water Splitting under Visible Light Irradiation. J. Photochem. Photobiol. C Photochem. Rev. 2010;11:179–209. doi: 10.1016/j.jphotochemrev.2011.02.003. - DOI
-
- Rahman M.Z., Davey K., Qiao S.Z. Carbon, Nitrogen and Phosphorus Containing Metal-Free Photocatalysts for Hydrogen Production: Progress and Challenges. J. Mater. Chem. A. 2018;6:1305–1322. doi: 10.1039/C7TA10404A. - DOI
-
- Sivaranjani K., Gopinath C.S. Porosity Driven Photocatalytic Activity of Wormhole Mesoporous TiO2-XNx in Direct Sunlight. J. Mater. Chem. 2011;21:2639–2647. doi: 10.1039/c0jm03825c. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

