A Specific Interaction between Ionic Liquids' Cations and Reichardt's Dye
- PMID: 36364035
- PMCID: PMC9653922
- DOI: 10.3390/molecules27217205
A Specific Interaction between Ionic Liquids' Cations and Reichardt's Dye
Abstract
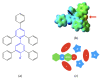
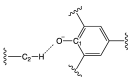
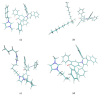
Solvatochromic probes are often used to understand solvation environments at the molecular scale. In the case of ionic liquids constituted by an anion and a cation, which are designed and paired in order to obtain a low melting point and other desirable physicochemical properties, these two indivisible components can interact in a very different way with the probe. This is the case with one of the most common probes: Reichardt's Dye. In the cases where the positive charge of the cation is delocalized on an aromatic ring such as imidazolium, the antibonding orbitals of the positively charged aromatic system are very similar in nature and energy to the LUMO of Reichardt's Dye. This leads to an interesting, specific cation-probe interaction that can be used to elucidate the nature of the ionic liquids' cations. Parallel computational and experimental investigations have been conducted to elucidate the nature of this interaction with respect to the molecular structure of the cation.
Keywords: Reichardt’s Dye; charge transfer; ionic liquids; molecular probes; solvatochromism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Welton T., Reichardt C. Solvents and Solvent Effects in Organic Chemistry. 4th ed. Wiley; Hoboken, NJ, USA: 2010.
-
- Reichardt C. Polarity of ionic liquids determined empirically by means of solvatochromic pyridinium N-phenolate betaine dyes. Green Chem. 2005;7:339. doi: 10.1039/b500106b. - DOI
-
- Reichardt C. Empirical Parameters of solvent polarity as linear free-energy relationships. Angew. Chem. Int. Ed. Engl. 1979;18:98–110. doi: 10.1002/anie.197900981. - DOI
-
- Reichardt C. Solvatochromic dyes as solvent polarity indicators. Chem Rev. 1994;94:2319–2358. doi: 10.1021/cr00032a005. - DOI
-
- Karmakar N.K., Pandey S., Pandey R.K., Shukla S.S. Solvatochromism: A tool for solvent discretion for UV-Vis spectroscopic studies. Appl. Spectrosc. Rev. 2021;56:513–529. doi: 10.1080/05704928.2020.1838918. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

