Multitargeted Molecular Docking and Dynamic Simulation Studies of Bioactive Compounds from Rosmarinus officinalis against Alzheimer's Disease
- PMID: 36364071
- PMCID: PMC9653785
- DOI: 10.3390/molecules27217241
Multitargeted Molecular Docking and Dynamic Simulation Studies of Bioactive Compounds from Rosmarinus officinalis against Alzheimer's Disease
Abstract
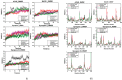
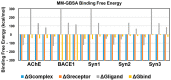
Alzheimer's disease (AD) has been associated with the hallmark features of cholinergic dysfunction, amyloid beta (Aβ) aggregation and impaired synaptic transmission, which makes the associated proteins, such as β-site amyloid precursor protein cleaving enzyme 1 (BACE I), acetylcholine esterase (AChE) and synapsin I, II and III, major targets for therapeutic intervention. The present study investigated the therapeutic potential of three major phytochemicals of Rosmarinus officinalis, ursolic acid (UA), rosmarinic acid (RA) and carnosic acid (CA), based on their binding affinity with AD-associated proteins. Detailed docking studies were conducted using AutoDock vina followed by molecular dynamic (MD) simulations using Amber 20. The docking analysis of the selected molecules showed the binding energies of their interaction with the target proteins, while MD simulations comprising root mean square deviation (RMSD), root mean square fluctuation (RMSF) and molecular mechanics/generalized born surface area (MM/GBSA) binding free energy calculations were carried out to check the stability of bound complexes. The drug likeness and the pharmacokinetic properties of the selected molecules were also checked through the Lipinski filter and ADMETSAR analysis. All these bioactive compounds demonstrated strong binding affinity with AChE, BACE1 and synapsin I, II and III. The results showed UA and RA to be potential inhibitors of AChE and BACE1, exhibiting binding energies comparable to those of donepezil, used as a positive control. The drug likeness and pharmacokinetic properties of these compounds also demonstrated drug-like characteristics, indicating the need for further in vitro and in vivo investigations to ascertain their therapeutic potential for AD.
Keywords: AChE; Alzheimer’s disease; BACE I; Rosmarinus officinalis; Synapsin I, II, III; docking.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Determination of Potential Lead Compound from Magnolia officinalis for Alzheimer's Disease through Pharmacokinetic Prediction, Molecular Docking, Dynamic Simulation, and Experimental Validation.Int J Mol Sci. 2024 Sep 29;25(19):10507. doi: 10.3390/ijms251910507. Int J Mol Sci. 2024. PMID: 39408835 Free PMC article.
-
Repurposing of phyto-ligand molecules from the honey bee products for Alzheimer's disease as novel inhibitors of BACE-1: small molecule bioinformatics strategies as amyloid-based therapy.Environ Sci Pollut Res Int. 2023 Apr;30(17):51143-51169. doi: 10.1007/s11356-023-25943-4. Epub 2023 Feb 20. Environ Sci Pollut Res Int. 2023. PMID: 36808033
-
BACE1 molecular docking and anti-Alzheimer's disease activities of ginsenosides.J Ethnopharmacol. 2016 Aug 22;190:219-30. doi: 10.1016/j.jep.2016.06.013. Epub 2016 Jun 5. J Ethnopharmacol. 2016. PMID: 27275774
-
Alzheimer's Disease and β-secretase Inhibition: An Update with a Focus on Computer-aided Inhibitor Design.Curr Drug Targets. 2022;23(3):266-285. doi: 10.2174/1389450122666210809100050. Curr Drug Targets. 2022. PMID: 34370634 Review.
-
Dual-target compounds for Alzheimer's disease: Natural and synthetic AChE and BACE-1 dual-inhibitors and their structure-activity relationship (SAR).Eur J Med Chem. 2021 Oct 5;221:113492. doi: 10.1016/j.ejmech.2021.113492. Epub 2021 Apr 24. Eur J Med Chem. 2021. PMID: 33984802 Review.
Cited by
-
Polyphenols Investigation and Antioxidant and Anticholinesterase Activities of Rosmarinus officinalis L. Species from Southwest Romania Flora.Molecules. 2024 Sep 18;29(18):4438. doi: 10.3390/molecules29184438. Molecules. 2024. PMID: 39339433 Free PMC article.
-
Role of Saponins from Platycodon grandiflorum in Alzheimer's Disease: DFT, Molecular Docking, and Simulation Studies in Key Enzymes.Molecules. 2025 Apr 17;30(8):1812. doi: 10.3390/molecules30081812. Molecules. 2025. PMID: 40333841 Free PMC article.
-
Determination of Potential Lead Compound from Magnolia officinalis for Alzheimer's Disease through Pharmacokinetic Prediction, Molecular Docking, Dynamic Simulation, and Experimental Validation.Int J Mol Sci. 2024 Sep 29;25(19):10507. doi: 10.3390/ijms251910507. Int J Mol Sci. 2024. PMID: 39408835 Free PMC article.
-
Ursolic acid and rosmarinic acid ameliorate alterations in hippocampal neurogenesis and social memory induced by amyloid beta in mouse model of Alzheimer's disease.Front Pharmacol. 2022 Dec 22;13:1058358. doi: 10.3389/fphar.2022.1058358. eCollection 2022. Front Pharmacol. 2022. PMID: 36618920 Free PMC article.
-
Computer-Aided Drug Design in Research on Chinese Materia Medica: Methods, Applications, Advantages, and Challenges.Pharmaceutics. 2025 Mar 1;17(3):315. doi: 10.3390/pharmaceutics17030315. Pharmaceutics. 2025. PMID: 40142979 Free PMC article. Review.
References
-
- Zhao J., Fu Y., Yasvoina M., Shao P., Hitt B., O’Connor T., Logan S., Maus E., Citron M., Berry R., et al. β-Site amyloid precursor protein cleaving enzyme 1 levels become elevated in neurons around amyloid plaques: Implications for Alzheimer’s disease pathogenesis. J. Neurosci. 2007;27:3639–3649. doi: 10.1523/JNEUROSCI.4396-06.2007. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

