Anthocyanins Formulated with Carboxymethyl Starch for Gastric and Intestinal Delivery
- PMID: 36364096
- PMCID: PMC9657027
- DOI: 10.3390/molecules27217271
Anthocyanins Formulated with Carboxymethyl Starch for Gastric and Intestinal Delivery
Abstract
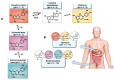
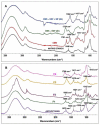
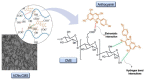
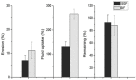
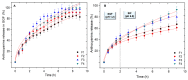
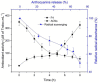
Anthocyanins obtained from jambolan have been used as active agents in different carboxymethyl starch-based tablet formulations and their release profiles evaluated in simulated gastric fluids (SGF) and simulated intestinal (SIF) fluids. Structural analysis highlighted a strong interaction between anthocyanins and carboxymethyl starch, evidenced by scanning electron microscopy and infrared analysis. Tablet dissolution behavior varied according to the pH of the media, being controlled by the swelling and/or erosion of the polymeric matrix. Various formulations for immediate, fast, and sustained release of anthocyanins for 30 min, 2 h and 12 h of dissolution have been developed. It was found that monolithic carboxymethyl starch tablets loaded with powdered jambolan extract efficiently afforded the complete delivery (100% of anthocyanins) to different sites of the simulated gastrointestinal tract and ensured the stability of these pigments, which maintained their antioxidant activity.
Keywords: Syzygium cumini; anthocyanins; bioactive agent delivery; carboxymethyl starch; monolithic tablets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Del Bò C., Ciappellano S., Klimis-Zacas D., Martini D., Gardana C., Riso P., Porrini M. Anthocyanin absorption, metabolism, and distribution from a wild blueberry-enriched diet (Vaccinium angustifolium) is affected by diet duration in the sprague-dawley rat. J. Agric. Food Chem. 2010;58:2491–2497. doi: 10.1021/jf903472x. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

