Development and Validation of a UHPLC-MS/MS-Based Method to Quantify Cenobamate in Human Plasma Samples
- PMID: 36364153
- PMCID: PMC9656984
- DOI: 10.3390/molecules27217325
Development and Validation of a UHPLC-MS/MS-Based Method to Quantify Cenobamate in Human Plasma Samples
Abstract
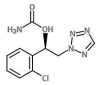
Cenobamate (CNB) is the newest antiseizure medication (ASM) approved by the FDA in 2019 to reduce uncontrolled partial-onset seizures in adult patients. Marketed as Xcopri in the USA or Ontozry in the EU (tablets), its mechanism of action has not been fully understood yet; however, it is known that it inhibits voltage-gated sodium channels and positively modulates the aminobutyric acid (GABA) ion channel. CNB shows 88% of oral bioavailability and is responsible for modifying the plasma concentrations of other co-administered ASMs, such as lamotrigine, carbamazepine, phenytoin, phenobarbital and the active metabolite of clobazam. It also interferes with CYP2B6 and CYP3A substrates. Nowadays, few methods are reported in the literature to quantify CNB in human plasma. The aim of this study was to develop and validate, according to the most recent guidelines, an analytical method using ultra-high-performance liquid chromatography coupled with tandem mass spectrometry (UHPLC-MS/MS) to evaluate CNB dosage in plasma samples. Furthermore, we provided a preliminary clinical application of our methodology by evaluating the pharmacokinetic parameters of CNB in two non-adult patients. Plasma levels were monitored for two months. Preliminary data showed a linear increase in plasma CNB concentrations, in both patients, in agreement with the increase in CNB dosage. A seizure-free state was reported for both patients at the dose of 150 mg per day.
Keywords: UHPLC–MS/MS; antiseizure medications; cenobamate; liquid chromatography; mass spectrometry; therapeutic drug monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


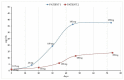
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

