Honokiol and Alpha-Mangostin Inhibit Mayaro Virus Replication through Different Mechanisms
- PMID: 36364188
- PMCID: PMC9659048
- DOI: 10.3390/molecules27217362
Honokiol and Alpha-Mangostin Inhibit Mayaro Virus Replication through Different Mechanisms
Abstract
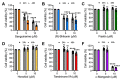
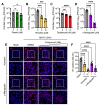
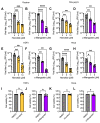
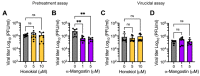
Mayaro virus (MAYV) is an emerging arbovirus with an increasing circulation across the Americas. In the present study, we evaluated the potential antiviral activity of the following natural compounds against MAYV and other arboviruses: Sanguinarine, (R)-Shikonin, Fisetin, Honokiol, Tanshinone IIA, and α-Mangostin. Sanguinarine and Shikonin showed significant cytotoxicity, whereas Fisetin, Honokiol, Tanshinone IIA, and α-Mangostin were well tolerated in all the cell lines tested. Honokiol and α-Mangostin treatment protected Vero-E6 cells against MAYV-induced damage and resulted in a dose-dependent reduction in viral progeny yields for each of the MAYV strains and human cell lines assessed. These compounds also reduced MAYV viral RNA replication in HeLa cells. In addition, Honokiol and α-Mangostin disrupted MAYV infection at different stages of the virus life cycle. Moreover, Honokiol and α-Mangostin decreased Una, Chikungunya, and Zika viral titers and downmodulated the expression of E1 and nsP1 viral proteins from MAYV, Una, and Chikungunya. Finally, in Honokiol- and α-Mangostin-treated HeLa cells, we observed an upregulation in the expression of type I interferon and specific interferon-stimulated genes, including IFNα, IFNβ, MxA, ISG15, OAS2, MDA-5, TNFα, and IL-1β, which may promote an antiviral cellular state. Our results indicate that Honokiol and α-Mangostin present potential broad-spectrum activity against different arboviruses through different mechanisms.
Keywords: Chikungunya; Honokiol; Mayaro; Una; Zika; antiviral activity; arboviruses; broad-spectrum activity; α-Mangostin.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Broad Antiviral Activity of Ginkgolic Acid against Chikungunya, Mayaro, Una, and Zika Viruses.Viruses. 2020 Apr 15;12(4):449. doi: 10.3390/v12040449. Viruses. 2020. PMID: 32326564 Free PMC article.
-
Targeting Host PIM Protein Kinases Reduces Mayaro Virus Replication.Viruses. 2022 Feb 18;14(2):422. doi: 10.3390/v14020422. Viruses. 2022. PMID: 35216015 Free PMC article.
-
Antiviral activity of silymarin against Mayaro virus and protective effect in virus-induced oxidative stress.Antiviral Res. 2018 Oct;158:8-12. doi: 10.1016/j.antiviral.2018.07.023. Epub 2018 Aug 1. Antiviral Res. 2018. PMID: 30076863
-
Mayaro Virus: The State-of-the-Art for Antiviral Drug Development.Viruses. 2022 Aug 16;14(8):1787. doi: 10.3390/v14081787. Viruses. 2022. PMID: 36016409 Free PMC article. Review.
-
Antiviral Drug Discovery and Development for Mayaro Fever - What do we have so far?Mini Rev Med Chem. 2020;20(10):921-928. doi: 10.2174/1389557520666200316160425. Mini Rev Med Chem. 2020. PMID: 32178610 Review.
Cited by
-
An update on the development of antiviral against Mayaro virus: from molecules to potential viral targets.Arch Microbiol. 2023 Mar 7;205(4):106. doi: 10.1007/s00203-023-03441-y. Arch Microbiol. 2023. PMID: 36881172 Free PMC article. Review.
-
Honokiol Inhibits SARS-CoV-2 Replication in Cell Culture at a Post-Entry Step.Microbiol Spectr. 2023 Jun 15;11(3):e0327322. doi: 10.1128/spectrum.03273-22. Epub 2023 May 4. Microbiol Spectr. 2023. PMID: 37212560 Free PMC article.
References
-
- Aguilar-Luis M.A., Del Valle-Mendoza J., Sandoval I., Silva-Caso W., Mazulis F., Carrillo-Ng H., Tarazona-Castro Y., Martins-Luna J., Aquino-Ortega R., Pena-Tuesta I., et al. A silent public health threat: Emergence of Mayaro virus and co-infection with Dengue in Peru. BMC Res. Notes. 2021;14:1–7. doi: 10.1186/s13104-021-05444-8. - DOI - PMC - PubMed
-
- Gonzalez-Escobar G., Churaman C., Rampersad C., Singh R., Nathaniel S. Mayaro virus detection in patients from rural and urban areas in Trinidad and Tobago during the Chikungunya and Zika virus outbreaks. Pathog. Glob. Health. 2021;115:188–195. doi: 10.1080/20477724.2021.1878445. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

