Surface Properties of Saponin-Chitosan Mixtures
- PMID: 36364333
- PMCID: PMC9658537
- DOI: 10.3390/molecules27217505
Surface Properties of Saponin-Chitosan Mixtures
Abstract
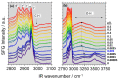
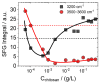
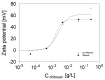
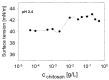
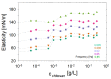
The surface properties of saponin and saponin-chitosan mixtures were analysed as a function of their bulk mixing ratio using vibrational sum-frequency generation (SFG), surface tensiometry and dilational rheology measurements. Our experiments show that saponin-chitosan mixtures present some remarkable properties, such as a strong amphiphilicity of the saponin and high dilational viscoelasticity. We believe this points to the presence of chitosan in the adsorption layer, despite its complete lack of surface activity. We explain this phenomenon by electrostatic interactions between the saponin as an anionic surfactant and chitosan as a polycation, leading to surface-active saponin-chitosan complexes and aggregates. Analysing the SFG intensity of the O-H stretching bands from interfacial water molecules, we found that in the case of pH 3.4 for a mixture consisting of 0.1 g/L saponin and 0.001 g/L chitosan, the adsorption layer was electrically neutral. This conclusion from SFG spectra is corroborated by results from surface tensiometry showing a significant reduction in surface tension and effects on the dilational surface elasticity strictly at saponin/chitosan ratios, where SFG spectra indicate zero net charge at the air-water interface.
Keywords: bio-compatible systems; biodegradable surfactants; chitosan; saponin; saponin—chitosan interactions.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
-
- Oleszek W., Hamed A. Saponin-Based Surfactants. In: Kjellin M., Johansson I., editors. Surfactants from Renewable Resources. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2010. pp. 239–249. - DOI
MeSH terms
Substances
Grants and funding
- 2016/21/B/ST8/02107/National Science Center
- "Biocompatible foams and emulsions stabilised by natural surfactants and particles for bio-medical application"/joint CNR-PAN (Jerzy Haber Institute of Catalysis and Surface Chemistry PAS and CNR-Institute of Condensed Matter Chemistry and Technologies for Energy) bilateral research project
- 2018-1-PL01-KA103-047692/Erasmus mobility project (MK)
- MAP project "Emulsion Dynamics and Droplet Interfaces - EDDI" European Space Agency contract n. 4000128643/19/NL/European Space Agency
LinkOut - more resources
Full Text Sources

