Unusual Formation of 1,2,4-Oxadiazine Core in Reaction of Amidoximes with Maleic or Fumaric Esters
- PMID: 36364335
- PMCID: PMC9655267
- DOI: 10.3390/molecules27217508
Unusual Formation of 1,2,4-Oxadiazine Core in Reaction of Amidoximes with Maleic or Fumaric Esters
Abstract
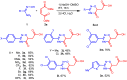
We have developed a simple and convenient method for the synthesis of 3-aryl- and 3-hetaryl-1,2,4-oxadiazin-5-ones bearing an easily functionalizable (methoxycarbonyl)methyl group at position 6 via the reaction of aryl or hetaryl amidoximes with maleates or fumarates. The conditions for this reaction were optimized. Different products can be synthesized selectively in good yields depending on the base used and the ratio of reactants: substituted (1,2,4-oxadiazin-6-yl)acetic acids, corresponding methyl esters, or hybrid 3-(aryl)-6-((3-(aryl)-1,2,4-oxadiazol-5-yl)methyl)-4H-1,2,4-oxadiazin-5(6H)-ones. The reaction is tolerant to substituents' electronic and steric effects in amidoximes. As a result, a series of 2-(5-oxo-3-(p-tolyl)-5,6-dihydro-4H-1,2,4-oxadiazin-6-yl)acetic acids, their methyl esters, and 1,2,4-oxadiazoles based on them were prepared and characterized by HRMS, 1H, and 13C NMR spectroscopy. The structures of three of them were elucidated with X-ray diffraction.
Keywords: amidoximes; basic medium; cyclization; esters; heterocycles; nucleophilic addition.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
-
- Baykov S., Semenov A., Tarasenko M., Boyarskiy V.P. Application of amidoximes for the heterocycles synthesis. Tetrahedron Lett. 2020;61:152403. doi: 10.1016/j.tetlet.2020.152403. - DOI
-
- Pivneva E.E., Galenko A.V., Dar’In D.V., Lobanov P.S. Rearrangement of the adducts of α-(aminocarbonyl)-acetamidoximes with acylacetylenes, leading to 2-aminopyrrole derivatives. Chem. Heterocycl. Compd. 2012;48:875–880. doi: 10.1007/s10593-012-1069-0. - DOI
-
- Shabalin D.A., Dunsford J.J., Ngwerume S., Saunders A.R., Gill D.M., Camp J.E. Synthesis of 2,4-Disubstituted Imidazoles via Nucleophilic Catalysis. Synlett. 2020;31:797–800. doi: 10.1055/s-0039-1690832. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

