Synthesis of Nanosilica for the Removal of Multicomponent Cd2+ and Cu2+ from Synthetic Water: An Experimental and Theoretical Study
- PMID: 36364357
- PMCID: PMC9658150
- DOI: 10.3390/molecules27217536
Synthesis of Nanosilica for the Removal of Multicomponent Cd2+ and Cu2+ from Synthetic Water: An Experimental and Theoretical Study
Abstract
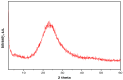
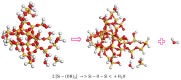
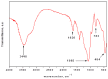
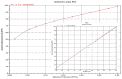
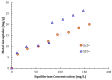
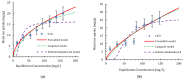
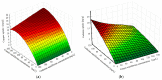
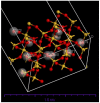
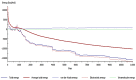
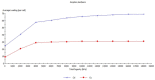
Copper and cadmium ions are among the top 120 hazardous chemicals listed by the Agency for Toxic Substances and Disease Registry (ATSDR) that can bind to organic and inorganic chemicals. Silica is one of the most abundant oxides that can limit the transport of these chemicals into water resources. Limited work has focused on assessing the applicability of nanosilica for the removal of multicomponent metal ions and studying their interaction on the surface of this adsorbent. Therefore, this study focuses on utilizing a nanosilica for the adsorption of Cd2+ and Cu2+ from water. Experimental work on the single- and multi-component adsorption of these ions was conducted and supported with theoretical interpretations. The nanosilica was characterized by its surface area, morphology, crystallinity, and functional groups. The BET surface area was 307.64 m2/g with a total pore volume of 4.95×10-3 cm3/g. The SEM showed an irregular amorphous shape with slits and cavities. Several Si-O-Si and hydroxyl groups were noticed on the surface of the silica. The single isotherm experiment showed that Cd2+ has a higher uptake (72.13 mg/g) than Cu2+ (29.28 mg/g). The multicomponent adsorption equilibrium shows an affinity for Cd2+ on the surface. This affinity decreases with increasing Cu2+ equilibrium concentration due to the higher isosteric heat from the interaction between Cd and the surface. The experimental data were modeled using isotherms for the single adsorption, with the Freundlich and the non-modified competitive Langmuir models showing the best fit. The molecular dynamics simulations support the experimental data where Cd2+ shows a multilayer surface coverage. This study provides insight into utilizing nanosilica for removing heavy metals from water.
Keywords: adsorption; cadmium; copper; heavy metals; multicomponent adsorption; nanosilica.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Al-Saida B., Amer W., Kandyel E.E., Ayad M.M. Enhanced dual catalytic activities of silver-polyaniline/titanium dioxide magnetic nanocomposite. J. Photochem. Photobiol. A Chem. 2020;392:112423. doi: 10.1016/j.jphotochem.2020.112423. - DOI
-
- Shawabkeh R., Rockstraw D.A., Bhada R.K. Copper and strontium adsorption by a novel carbon material manufactured from pecan shells. Carbon. 2002;40:781–786. doi: 10.1016/S0008-6223(01)00198-1. - DOI
-
- Zahir A., Aslam Z., Kamal M.S., Ahmad W., Abbas A., Shawabkeh R.A. Development of novel cross-linked chitosan for the removal of anionic Congo red dye. J. Mol. Liq. 2017;244:211–218. doi: 10.1016/j.molliq.2017.09.006. - DOI
-
- Shawabkeh R., Al-Harahsheh A., Al-Otoom A. Copper and zinc sorption by treated oil shale ash. Sep. Purif. Technol. 2004;40:251–257. doi: 10.1016/j.seppur.2004.03.006. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

