2-Imidazoline Nitroxide Derivatives of Cymantrene
- PMID: 36364372
- PMCID: PMC9659262
- DOI: 10.3390/molecules27217545
2-Imidazoline Nitroxide Derivatives of Cymantrene
Abstract
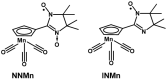
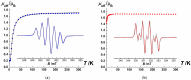
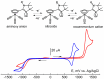
The 2-imidazoline nitroxide derivatives of cymantrene-2-(η5-cyclopentadienyl)tricarbonylmanganese(I)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-3-oxide-1-oxyl (NNMn) and 2-(η5-cyclopentadienyl)tricarbonylmanganese(I)-4,4,5,5-tetramethyl-4,5-dihydro-1H-imidazole-1-oxyl (INMn) were synthesized. It was shown that NNMn and INMn exhibit a sufficiently high kinetic stability both in solids and in solutions under normal conditions. Their structural characteristics, magnetic properties and electrochemical behavior are close to Re(I) analogs. This opens the prospect of using paramagnetic cymantrenes as prototypes in the design of Re(I) half-sandwiched derivatives for theranostics, where therapy is combined with diagnostics by magnetic resonance imaging due to the contrast properties of nitroxide radicals.
Keywords: cymantrene; manganeseorganic; nitroxide; paramagnets.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Ovcharenko V. 13. Metal-Nitroxide Complexes: Synthesis and Magnetostructural Correlations. In: Hicks R.G., editor. Radicals: Fundamentals and Applied Aspects of Odd Electron Compounds. John Wiley & Sons, Ltd.; Hoboken, NJ, USA: 2010. pp. 461–506.
-
- Tretyakov E.V., Ovcharenko V.I., Terent’ev A.O., Krylov I.B., Magdesieva T.V., Mazhukin D.G., Gritsan N.P. Conjugated nitroxides. Russ. Chem. Rev. 2022;91:RCR5025. doi: 10.1070/RCR5025. - DOI
-
- Tretyakov E.V., Ovcharenko V.I. The chemistry of nitroxide radicals in the molecular design of magnets. Russ. Chem. Rev. 2009;78:971–1012. doi: 10.1070/RC2009v078n11ABEH004093. - DOI
-
- Boocock D.G.B., Darcy R., Ullman E.F. Studies of Free Radicals. II. Chemical Properties of Nitronylnitroxides. A Unique Radical Anion. J. Am. Chem. Soc. 1968;90:5945–5946. doi: 10.1021/ja01023a078. - DOI
-
- Tretyakov E.V., Romanenko G.V., Stass D.V., Mareev A.V., Medvedeva A.S., Ovcharenko V.I. Key influence of the nature of the substituent in the propynal molecule on the outcome of its reaction with vicinal di(N-hydroxyamine) Russ. Chem. Bull. 2008;57:601–607. doi: 10.1007/s11172-008-0094-8. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

